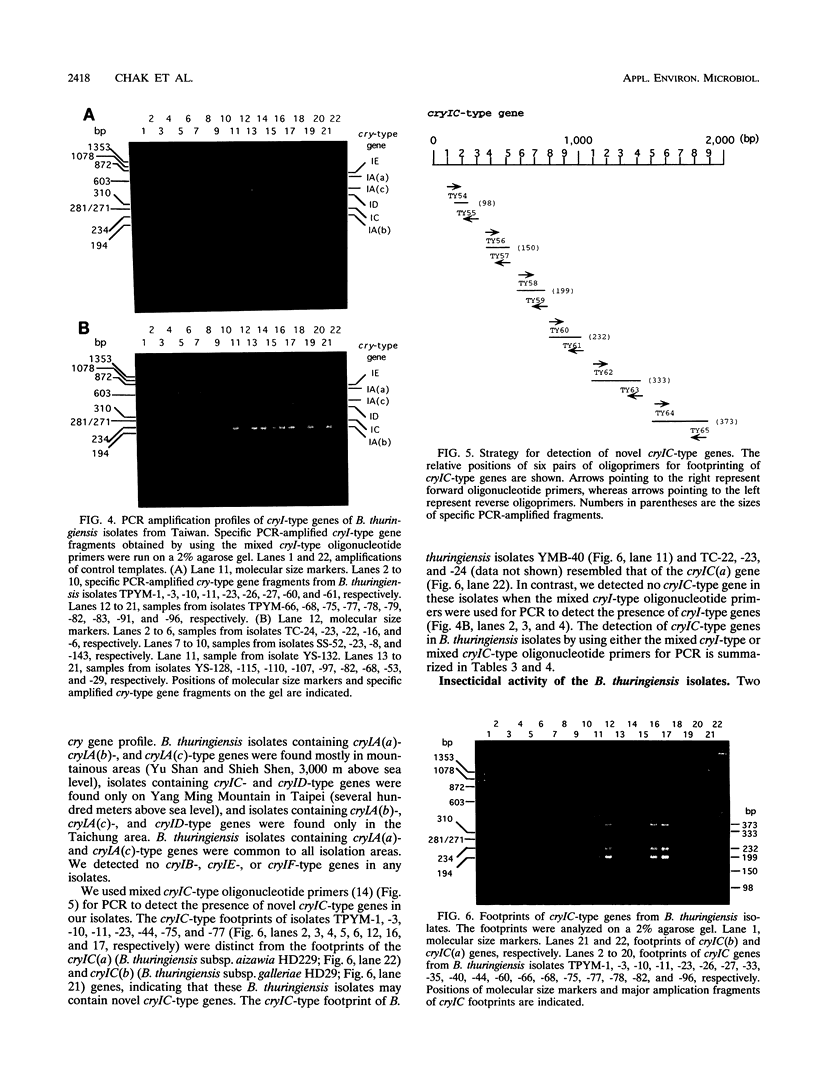
Abstract
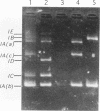
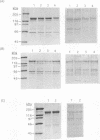
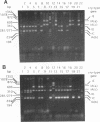
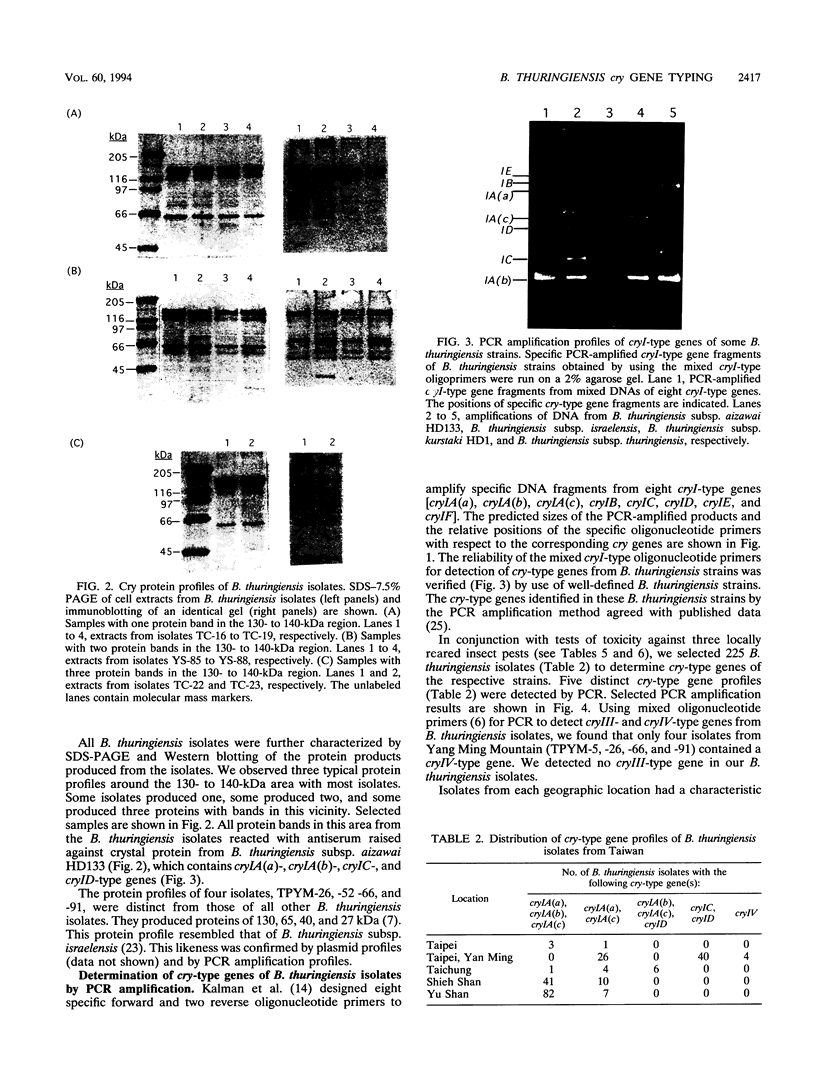
Using PCR with a set of specific oligonucleotide primers to detect cryI-type genes, we were able to screen the cry-type genes of 225 Bacillus thuringiensis soil isolates from Taiwan without much cost in time or labor. Some combinations of cry genes (the cry-type profile) in a single isolate were unique. We identified five distinct profiles of crystal genes from the B. thuringiensis soil isolates from Taiwan. The cry genes included cryIA(a), cryIA(b), cryIA(c), cryIC, cryID, and cryIV. Interestingly, 501 B. thuringiensis isolates (93.5% of the total number that we identified) were isolated from areas at high altitudes. The profiles of cry-type genes were distinct in all isolation areas. The distribution of cry-type genes of our isolates therefore depended on geography. Using PCR footprinting to detect cryIC-type genes, we identified two distinct cryIC footprints from some of our isolates, indicating that these isolates may contain novel cryIC-type genes. B. thuringiensis isolates containing cryIA(a)-, cryIA(b)-, and cryIA(c)-type genes exhibited much greater activity against Plutella xylostella than did other isolates, indicating that multiple cry-type genes may be used as markers for the prediction of insecticidal activities.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. E., Jr, Faust R. M., Wabiko H., Raymond K. C., Bulla L. A., Jr The biotechnology of Bacillus thuringiensis. Crit Rev Biotechnol. 1987;6(2):163–232. doi: 10.3109/07388558709113596. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Bechtel D. B., Kramer K. J., Shethna Y. I., Aronson A. I., Fitz-James P. C. Ultrastructure, physiology, and biochemistry of Bacillus thuringiensis. Crit Rev Microbiol. 1980;8(2):147–204. doi: 10.3109/10408418009081124. [DOI] [PubMed] [Google Scholar]
- Carozzi N. B., Kramer V. C., Warren G. W., Evola S., Koziel M. G. Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl Environ Microbiol. 1991 Nov;57(11):3057–3061. doi: 10.1128/aem.57.11.3057-3061.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak K. F., Young Y. M. Characterization of the Bacillus thuringiensis strains isolated from Taiwan. Proc Natl Sci Counc Repub China B. 1990 Jul;14(3):175–182. [PubMed] [Google Scholar]
- González J. M., Jr, Carlton B. C. Patterns of plasmid DNA in crystalliferous and acrystalliferous strains of Bacillus thuringiensis. Plasmid. 1980 Jan;3(1):92–98. doi: 10.1016/s0147-619x(80)90038-4. [DOI] [PubMed] [Google Scholar]
- Honée G., van der Salm T., Visser B. Nucleotide sequence of crystal protein gene isolated from B. thuringiensis subspecies entomocidus 60.5 coding for a toxin highly active against Spodoptera species. Nucleic Acids Res. 1988 Jul 11;16(13):6240–6240. doi: 10.1093/nar/16.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman S., Kiehne K. L., Libs J. L., Yamamoto T. Cloning of a novel cryIC-type gene from a strain of Bacillus thuringiensis subsp. galleriae. Appl Environ Microbiol. 1993 Apr;59(4):1131–1137. doi: 10.1128/aem.59.4.1131-1137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. A., Travers R. S. Worldwide Abundance and Distribution of Bacillus thuringiensis Isolates. Appl Environ Microbiol. 1989 Oct;55(10):2437–2442. doi: 10.1128/aem.55.10.2437-2442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser B., Munsterman E., Stoker A., Dirkse W. G. A novel Bacillus thuringiensis gene encoding a Spodoptera exigua-specific crystal protein. J Bacteriol. 1990 Dec;172(12):6783–6788. doi: 10.1128/jb.172.12.6783-6788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. S., Ellar D. J. Cloning and expression of two homologous genes of Bacillus thuringiensis subsp. israelensis which encode 130-kilodalton mosquitocidal proteins. J Bacteriol. 1988 Feb;170(2):727–735. doi: 10.1128/jb.170.2.727-735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]