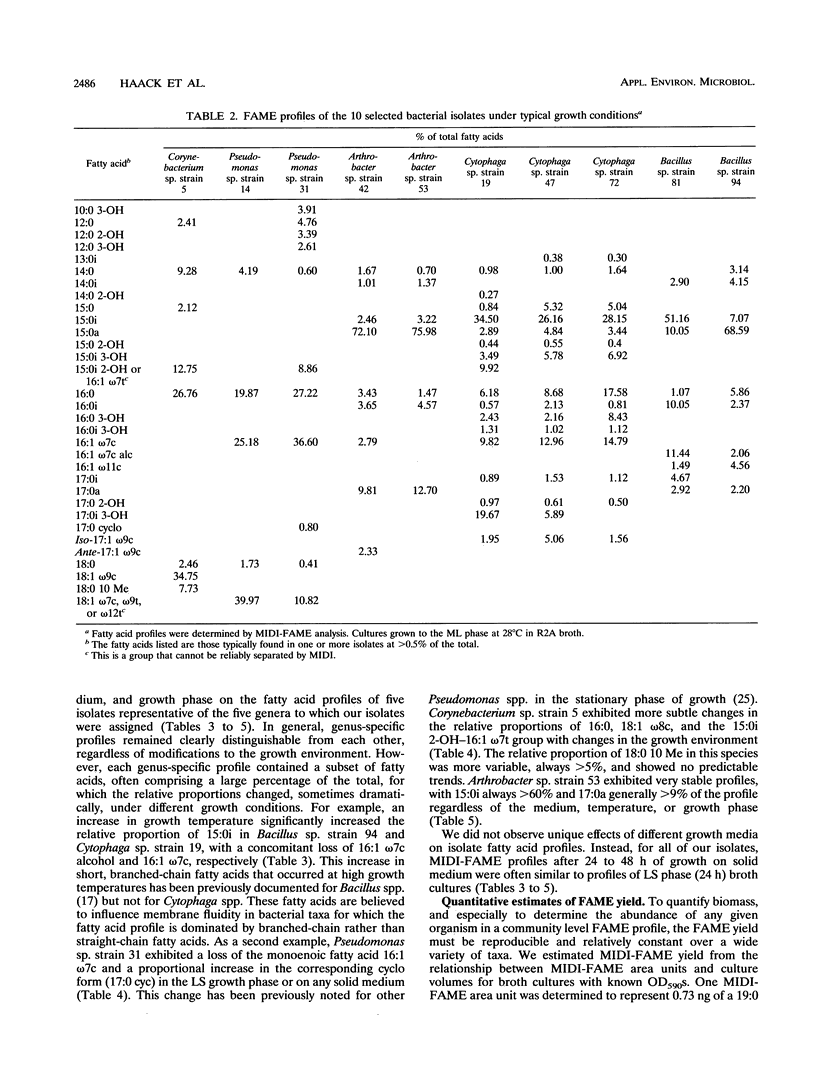
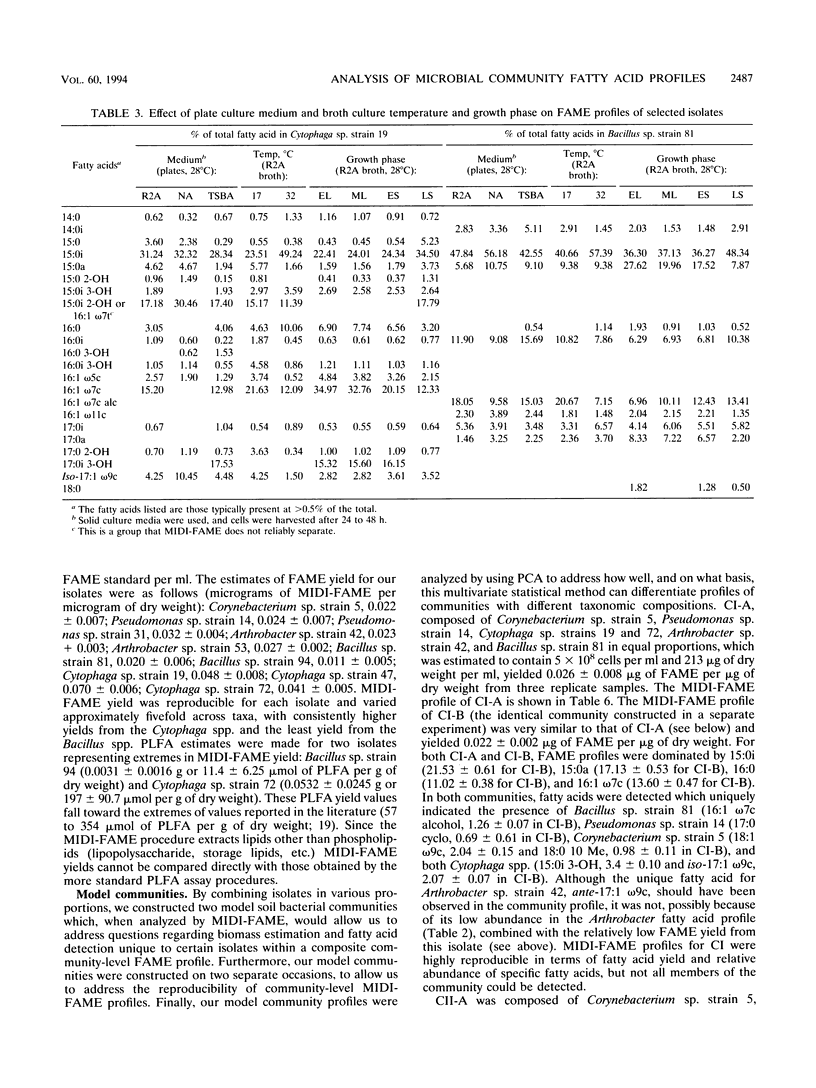
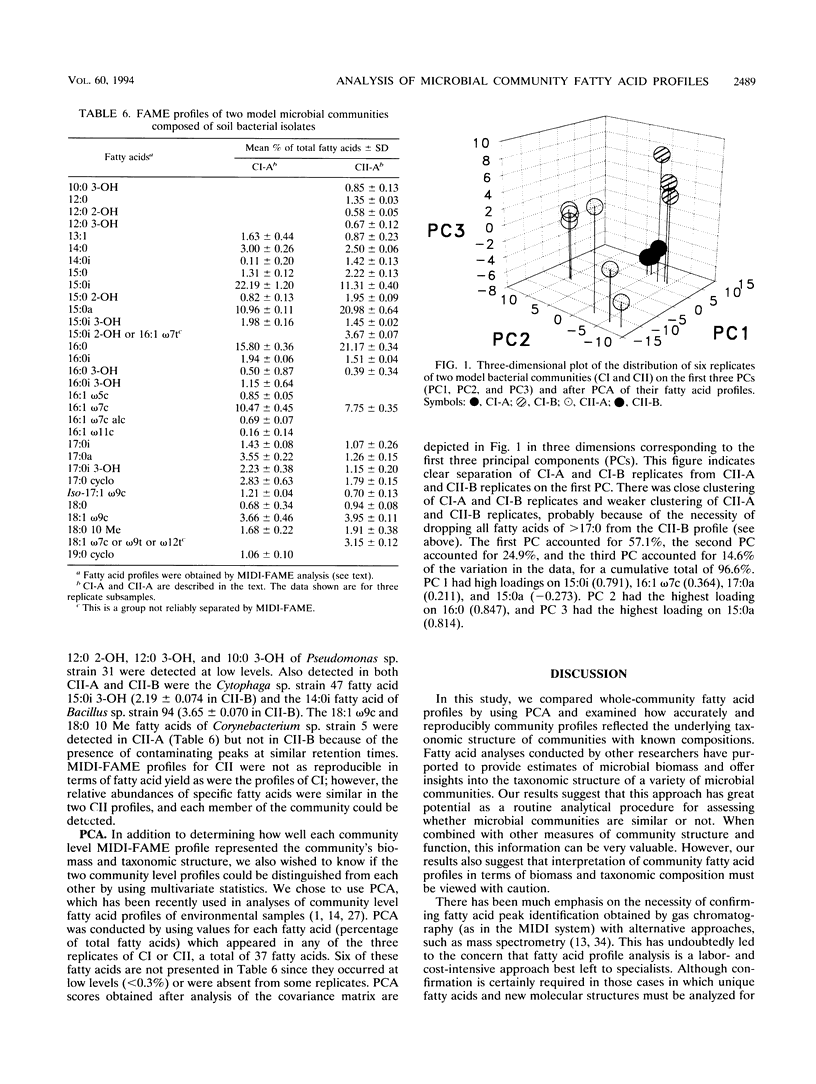
Abstract
We determined the accuracy and reproducibility of whole-community fatty acid methyl ester (FAME) analysis with two model bacterial communities differing in composition by using the Microbial ID, Inc. (MIDI), system. The biomass, taxonomic structure, and expected MIDI-FAME profiles under a variety of environmental conditions were known for these model communities a priori. Not all members of each community could be detected in the composite profile because of lack of fatty acid “signatures” in some isolates or because of variations (approximately fivefold) in fatty acid yield across taxa. MIDI-FAME profiles of replicate subsamples of a given community were similar in terms of fatty acid yield per unit of community dry weight and relative proportions of specific fatty acids. Principal-components analysis (PCA) of MIDI-FAME profiles resulted in a clear separation of the two different communities and a clustering of replicates of each community from two separate experiments on the first PCA axis. The first PCA axis accounted for 57.1% of the variance in the data and was correlated with fatty acids that varied significantly between communities and reflected the underlying community taxonomic structure. On the basis of our data, community fatty acid profiles can be used to assess the relative similarities and differences of microbial communities that differ in taxonomic composition. However, detailed interpretation of community fatty acid profiles in terms of biomass or community taxonomic composition must be viewed with caution until our knowledge of the quantitative and qualitative distribution of fatty acids over a wide variety of taxa and the effects of growth conditions on fatty acid profiles is more extensive.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achenbach H. The pigments of Flexibacter elegans: novel and chemosystematically useful compounds. Arch Microbiol. 1974;101(2):131–144. doi: 10.1007/BF00455933. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bai J., Ying K., Jaron D. Cardiovascular responses to external counterpulsation: a computer simulation. Med Biol Eng Comput. 1992 May;30(3):317–323. doi: 10.1007/BF02446970. [DOI] [PubMed] [Google Scholar]
- Bobbie R. J., White D. C. Characterization of benthic microbial community structure by high-resolution gas chromatography of Fatty Acid methyl esters. Appl Environ Microbiol. 1980 Jun;39(6):1212–1222. doi: 10.1128/aem.39.6.1212-1222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båth E., Frostegård A., Fritze H. Soil Bacterial Biomass, Activity, Phospholipid Fatty Acid Pattern, and pH Tolerance in an Area Polluted with Alkaline Dust Deposition. Appl Environ Microbiol. 1992 Dec;58(12):4026–4031. doi: 10.1128/aem.58.12.4026-4031.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckert J. B., Hood M. A., White D. C. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol. 1986 Oct;52(4):794–801. doi: 10.1128/aem.52.4.794-801.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluzny M. A., Duncan L. A., Merritt M. V., Epps D. E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985 Jan;26(1):135–140. [PubMed] [Google Scholar]
- Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991 Jun;55(2):288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Rajendran N., Matsuda O., Imamura N., Urushigawa Y. Variation in microbial biomass and community structure in sediments of eutrophic bays as determined by phospholipid ester-linked Fatty acids. Appl Environ Microbiol. 1992 Feb;58(2):562–571. doi: 10.1128/aem.58.2.562-571.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunlid A., Hoitink H. A., Low C., White D. C. Characterization of bacteria that suppress rhizoctonia damping-off in bark compost media by analysis of Fatty Acid biomarkers. Appl Environ Microbiol. 1989 Jun;55(6):1368–1374. doi: 10.1128/aem.55.6.1368-1374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., White D. C. Lipid analysis in microbial ecology: quantitative approaches to the study of microbial communities. Bioscience. 1989 Sep;39(8):535–541. [PubMed] [Google Scholar]


