Abstract
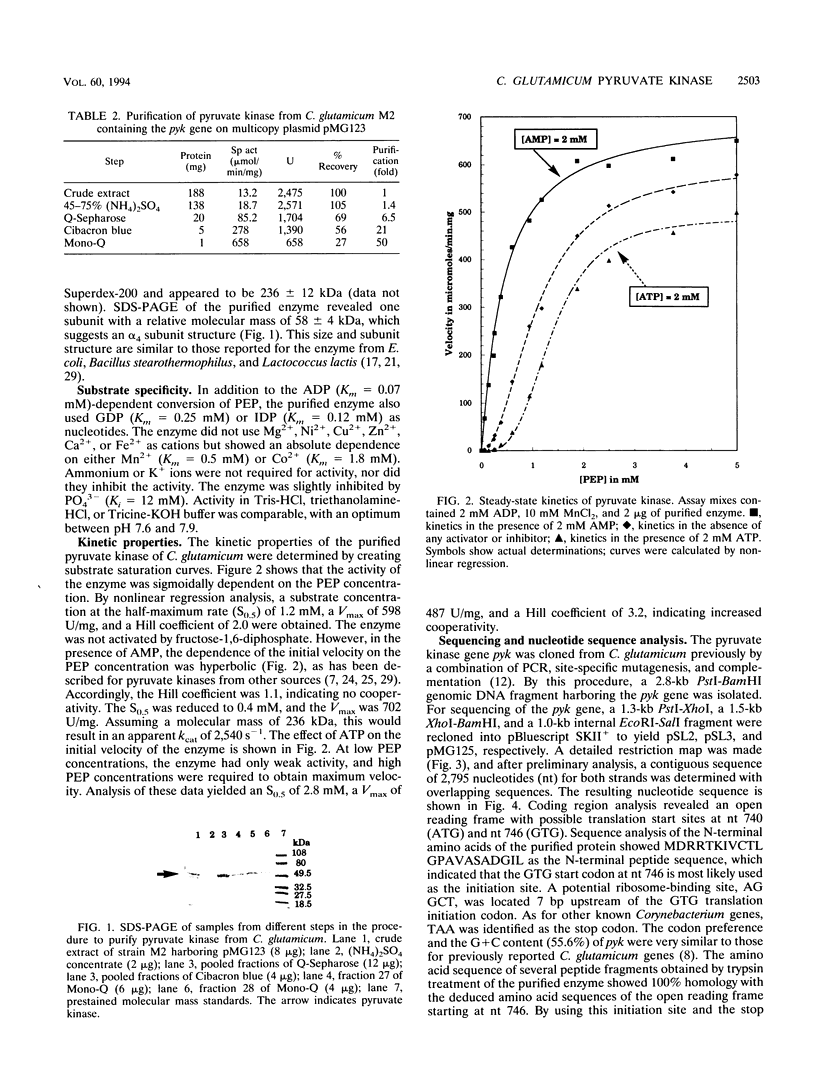
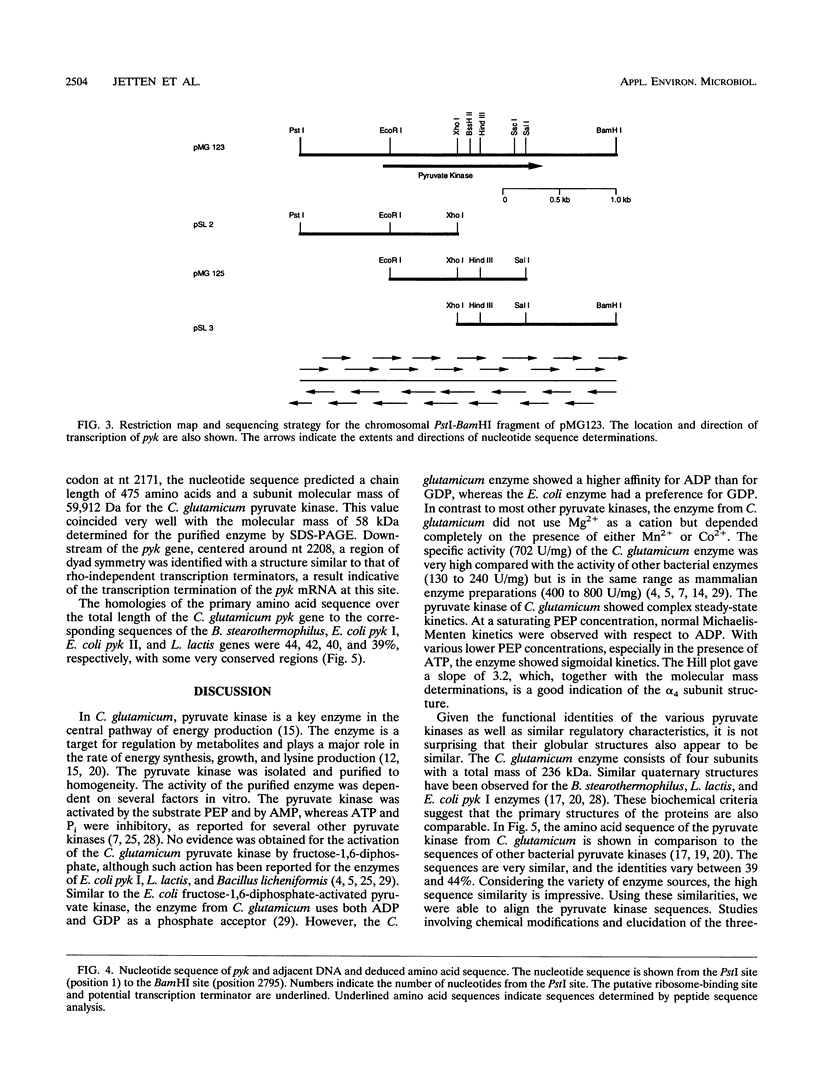
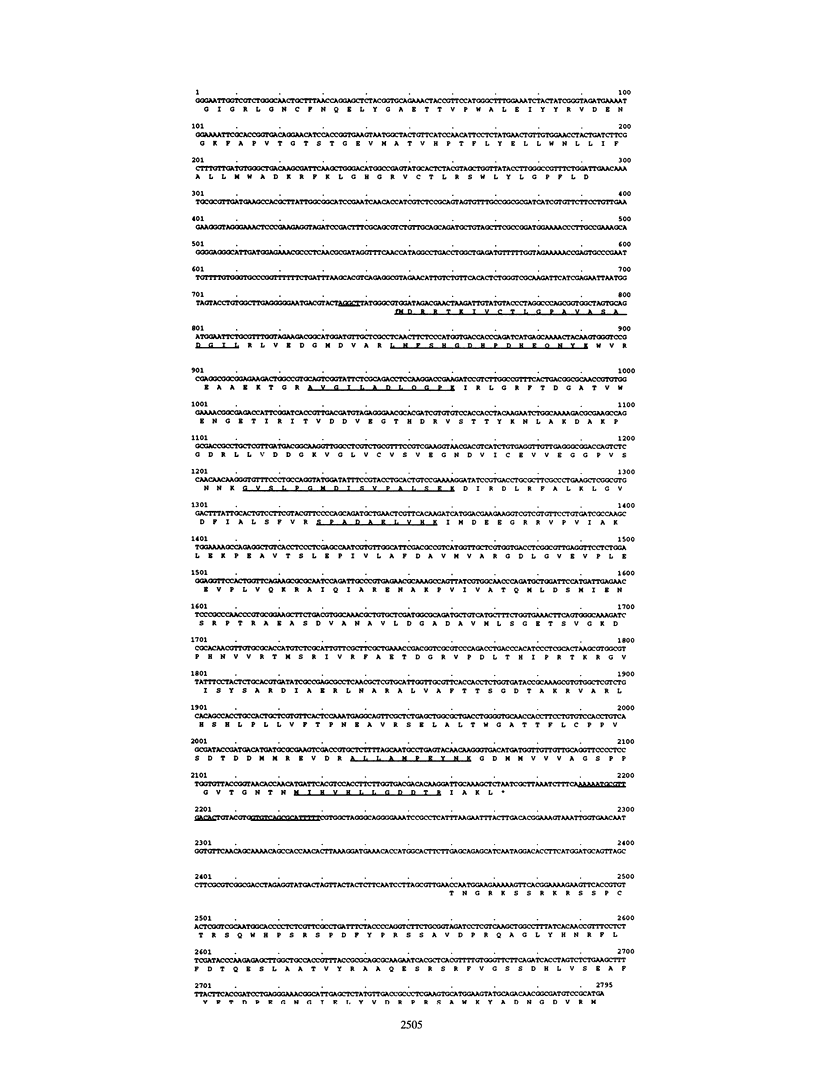
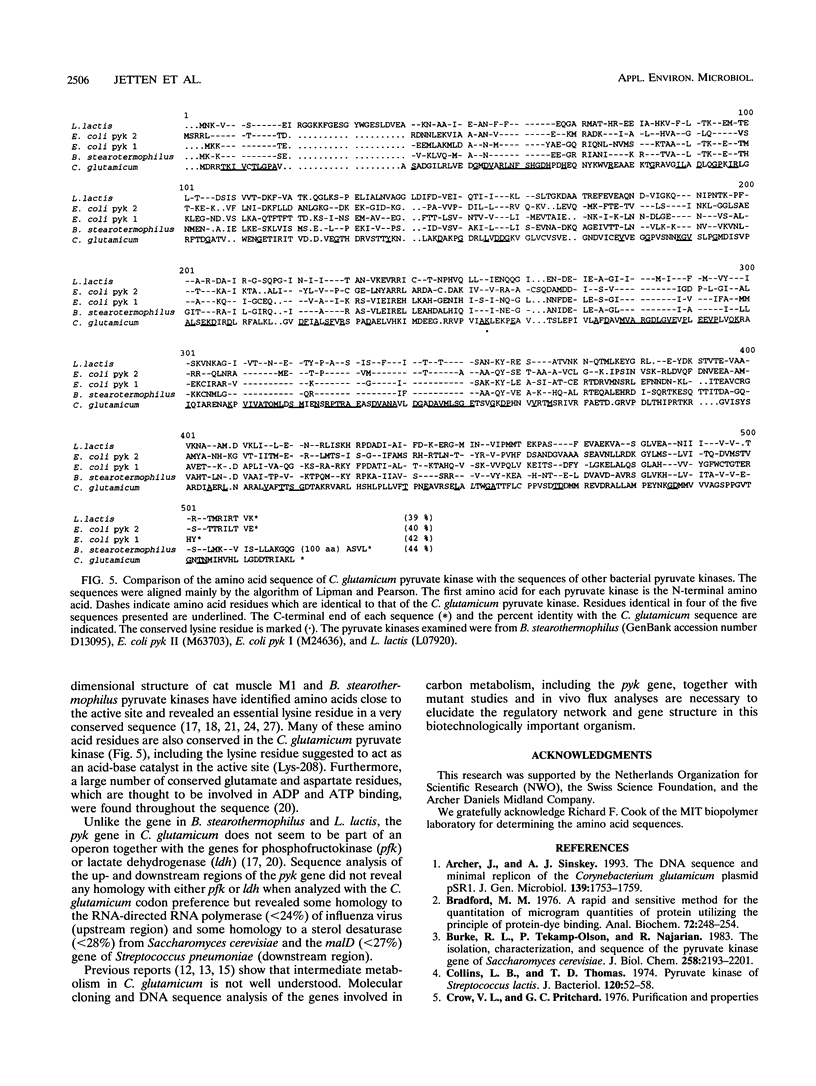
Pyruvate kinase activity is an important element in the flux control of the intermediate metabolism. The purified enzyme from Corynebacterium glutamicum demonstrated a marked sigmoidal dependence of the initial rate on the phosphoenolpyruvate concentration. In the presence of the negative allosteric effector ATP, the phosphoenolpyruvate concentration at the half-maximum rate (S0.5) increased from 1.2 to 2.8 mM, and cooperation, as expressed by the Hill coefficient, increased from 2.0 to 3.2. AMP promoted opposite effects: the S0.5 was decreased to 0.4 mM, and the enzyme exhibited almost no cooperation. The maximum reaction rate was 702 U/mg, which corresponded to an apparent kcat of 2,540 s-1. The enzyme was not influenced by fructose-1,6-diphosphate and used Mn2+ or Co2+ as cations. Sequence determination of the C. glutamicum pyk gene revealed an open reading frame coding for a polypeptide of 475 amino acids. From this information and the molecular mass of the native protein, it follows that the pyruvate kinase is a tetramer of 236 kDa. Comparison of the deduced polypeptide sequence with the sequences of other bacterial pyruvate kinases showed 39 to 44% homology, with some regions being very strongly conserved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. A., Sinskey A. J. The DNA sequence and minimal replicon of the Corynebacterium glutamicum plasmid pSR1: evidence of a common ancestry with plasmids from C. diphtheriae. J Gen Microbiol. 1993 Aug;139(8):1753–1759. doi: 10.1099/00221287-139-8-1753. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Tekamp-Olson P., Najarian R. The isolation, characterization, and sequence of the pyruvate kinase gene of Saccharomyces cerevisiae. J Biol Chem. 1983 Feb 25;258(4):2193–2201. [PubMed] [Google Scholar]
- Collins L. B., Thomas T. D. Pyruvate kinase of Streptococcus lactis. J Bacteriol. 1974 Oct;120(1):52–58. doi: 10.1128/jb.120.1.52-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Purification and properties of pyruvate kinase from Streptococcus lactis. Biochim Biophys Acta. 1976 Jun 7;438(1):90–101. doi: 10.1016/0005-2744(76)90225-4. [DOI] [PubMed] [Google Scholar]
- Diesterhaft M., Freese E. Pyruvate kinase of bacillus subtilis. Biochim Biophys Acta. 1972 May 12;268(2):373–380. doi: 10.1016/0005-2744(72)90332-4. [DOI] [PubMed] [Google Scholar]
- Eikmanns B. J. Identification, sequence analysis, and expression of a Corynebacterium glutamicum gene cluster encoding the three glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase, 3-phosphoglycerate kinase, and triosephosphate isomerase. J Bacteriol. 1992 Oct;174(19):6076–6086. doi: 10.1128/jb.174.19.6076-6086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follettie M. T., Peoples O. P., Agoropoulou C., Sinskey A. J. Gene structure and expression of the Corynebacterium flavum N13 ask-asd operon. J Bacteriol. 1993 Jul;175(13):4096–4103. doi: 10.1128/jb.175.13.4096-4103.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Genetics and intermediary metabolism. Annu Rev Genet. 1992;26:159–177. doi: 10.1146/annurev.ge.26.120192.001111. [DOI] [PubMed] [Google Scholar]
- Garcia-Olalla C., Garrido-Pertierra A. Purification and kinetic properties of pyruvate kinase isoenzymes of Salmonella typhimurium. Biochem J. 1987 Jan 15;241(2):573–581. doi: 10.1042/bj2410573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler M., Jetten M., Lee S. H., Sinskey A. J. Cloning of the pyruvate kinase gene (pyk) of Corynebacterium glutamicum and site-specific inactivation of pyk in a lysine-producing Corynebacterium lactofermentum strain. Appl Environ Microbiol. 1994 Jul;60(7):2494–2500. doi: 10.1128/aem.60.7.2494-2500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E. R., Cottam G. L. Isozymes of pyruvate kinase in vertebrates: their physical, chemical, kinetic and immunological properties. Int J Biochem. 1978;9(11):785–793. doi: 10.1016/0020-711x(78)90027-7. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Llanos R. M., Harris C. J., Hillier A. J., Davidson B. E. Identification of a novel operon in Lactococcus lactis encoding three enzymes for lactic acid synthesis: phosphofructokinase, pyruvate kinase, and lactate dehydrogenase. J Bacteriol. 1993 May;175(9):2541–2551. doi: 10.1128/jb.175.9.2541-2551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead H., Clayden D. A., Barford D., Lorimer C. G., Fothergill-Gilmore L. A., Schiltz E., Schmitt W. The structure of cat muscle pyruvate kinase. EMBO J. 1986 Mar;5(3):475–481. doi: 10.1002/j.1460-2075.1986.tb04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara O., Dorit R. L., Gilbert W. Direct genomic sequencing of bacterial DNA: the pyruvate kinase I gene of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6883–6887. doi: 10.1073/pnas.86.18.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. II. Regulation of phosphoenolpyruvate carboxylase and pyruvate kinase. J Biochem. 1969 Sep;66(3):297–311. doi: 10.1093/oxfordjournals.jbchem.a129148. [DOI] [PubMed] [Google Scholar]
- Sakai H., Ohta T. Molecular cloning and nucleotide sequence of the gene for pyruvate kinase of Bacillus stearothermophilus and the production of the enzyme in Escherichia coli. Evidence that the genes for phosphofructokinase and pyruvate kinase constitute an operon. Eur J Biochem. 1993 Feb 1;211(3):851–859. doi: 10.1111/j.1432-1033.1993.tb17618.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza M. L., Valentini G., Malcovati M. Fructose-1,6-bisphosphate-activated pyruvate kinase from Escherichia coli. Nature of bonds involved in the allosteric mechanism. Eur J Biochem. 1990 Aug 17;191(3):701–704. doi: 10.1111/j.1432-1033.1990.tb19178.x. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. II. Kinetic properties. J Biol Chem. 1971 Mar 25;246(6):1746–1755. [PubMed] [Google Scholar]
- Vallino J. J., Stephanopoulos G. Metabolic flux distributions in Corynebacterium glutamicum during growth and lysine overproduction. Biotechnol Bioeng. 1993 Mar 15;41(6):633–646. doi: 10.1002/bit.260410606. [DOI] [PubMed] [Google Scholar]
- Walker D., Chia W. N., Muirhead H. Key residues in the allosteric transition of Bacillus stearothermophilus pyruvate kinase identified by site-directed mutagenesis. J Mol Biol. 1992 Nov 5;228(1):265–276. doi: 10.1016/0022-2836(92)90505-e. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Rayman M. K., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. II. Effectors and regulatory properties of the enzyme activated by ribose 5-phosphate. Can J Biochem. 1975 Apr;53(4):444–454. doi: 10.1139/o75-061. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. I. Physicochemical and regulatory properties of the enzyme activated by fructose 1,6-diphosphate. J Biol Chem. 1974 Jan 10;249(1):265–274. [PubMed] [Google Scholar]
- de Graaff L., van den Broeck H., Visser J. Isolation and characterization of the Aspergillus niger pyruvate kinase gene. Curr Genet. 1992 Jul;22(1):21–27. doi: 10.1007/BF00351737. [DOI] [PubMed] [Google Scholar]



