Abstract
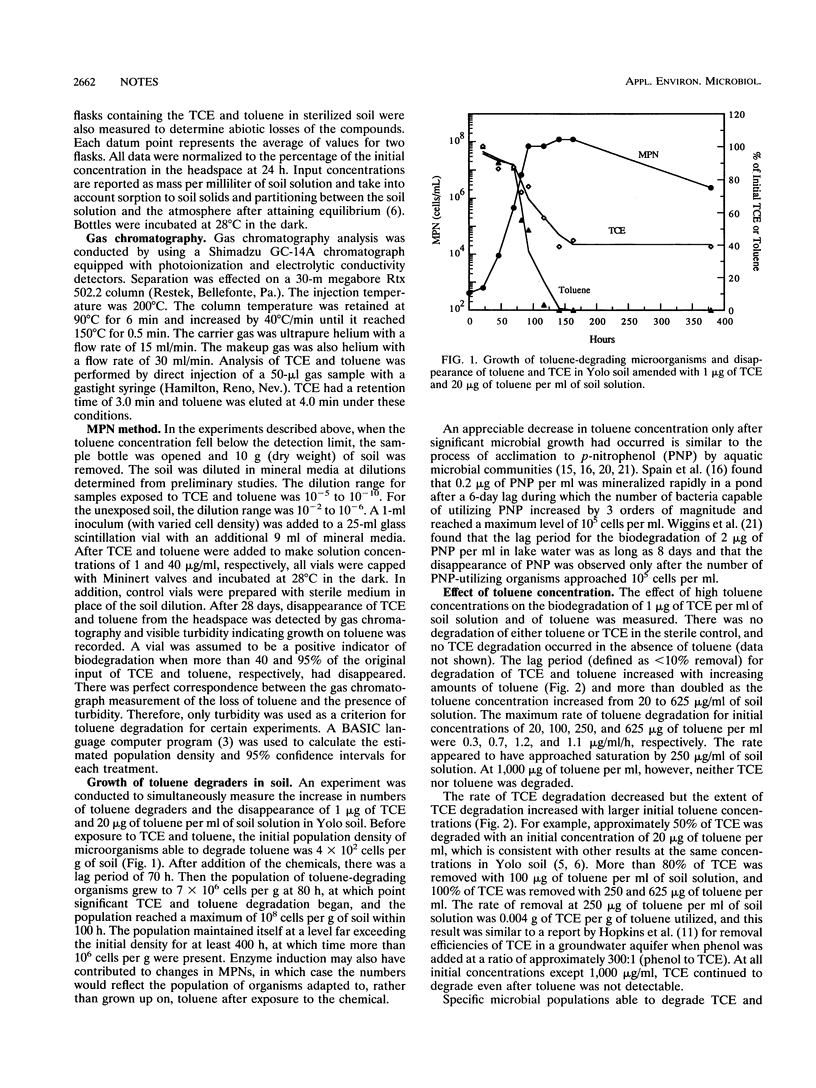
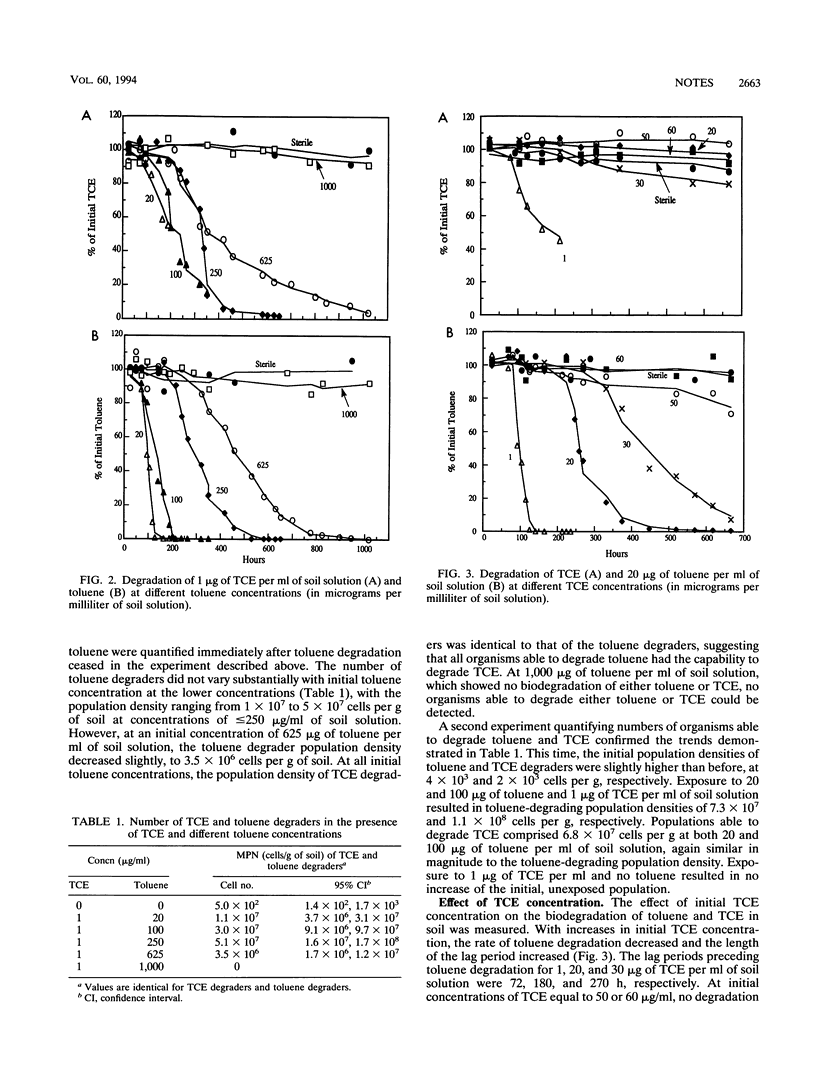
Toluene is one of several cosubstrates able to support the cometabolism of trichloroethylene (TCE) by soil microbial communities. Indigenous microbial populations in soil degraded TCE in the presence, but not the absence, of toluene after a 60- to 80-h lag period. Initial populations of toluene and TCE degraders ranged from 0.2 x 10(3) to 4 x 10(3) cells per g of soil and increased by more than 4 orders of magnitude after the addition of 20 micrograms of toluene and 1 microgram of TCE per ml of soil solution. The numbers of TCE and toluene degraders and the percent removal of TCE increased with an increase in initial toluene concentration. As the initial TCE concentration was increased from 1 to 20 micrograms/ml, the numbers of toluene and TCE degraders and the rate of toluene degradation decreased, and no TCE degradation occurred. No toluene or TCE degradation occurred at a TCE concentration of 50 micrograms/ml.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Cohen L., McCarty P. L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991 Jan;57(1):228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broholm Kim, Jensen Bjørn K., Christensen Thomas H., Olsen Lajla. Toxicity of 1,1,1-Trichloroethane and Trichloroethene on a Mixed Culture of Methane-Oxidizing Bacteria. Appl Environ Microbiol. 1990 Aug;56(8):2488–2493. doi: 10.1128/aem.56.8.2488-2493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D. Biochemical diversity of trichloroethylene metabolism. Annu Rev Microbiol. 1991;45:283–299. doi: 10.1146/annurev.mi.45.100191.001435. [DOI] [PubMed] [Google Scholar]
- Fan S., Scow K. M. Biodegradation of trichloroethylene and toluene by indigenous microbial populations in soil. Appl Environ Microbiol. 1993 Jun;59(6):1911–1918. doi: 10.1128/aem.59.6.1911-1918.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Phelps T. J., Ringelberg D., Mikell A. T., White D. C. Mineralization of trichloroethylene by heterotrophic enrichment cultures. Appl Environ Microbiol. 1988 Jul;54(7):1709–1714. doi: 10.2172/666263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom B. R., Chapman P. J., Pritchard P. H. Phenol and trichloroethylene degradation by Pseudomonas cepacia G4: kinetics and interactions between substrates. Appl Environ Microbiol. 1990 May;56(5):1279–1285. doi: 10.1128/aem.56.5.1279-1285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. M., Grbić-Galić D. Influence of endogenous and exogenous electron donors and trichloroethylene oxidation toxicity on trichloroethylene oxidation by methanotrophic cultures from a groundwater aquifer. Appl Environ Microbiol. 1991 Jan;57(1):236–244. doi: 10.1128/aem.57.1.236-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins G. D., Semprini L., McCarty P. L. Microcosm and in situ field studies of enhanced biotransformation of trichloroethylene by phenol-utilizing microorganisms. Appl Environ Microbiol. 1993 Jul;59(7):2277–2285. doi: 10.1128/aem.59.7.2277-2285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Oedzes J. Y., van der Waarde J. J., Janssen D. B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporium OB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991 Jan;57(1):7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Vink R. L., Janssen D. B., Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989 Nov;55(11):2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A., Monti C. A., Pritchard P. H., Cripe C. R. Comparison of p-Nitrophenol Biodegradation in Field and Laboratory Test Systems. Appl Environ Microbiol. 1984 Nov;48(5):944–950. doi: 10.1128/aem.48.5.944-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988 Jul;54(7):1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Householder S. R. Toxicity of Trichloroethylene to Pseudomonas putida F1 Is Mediated by Toluene Dioxygenase. Appl Environ Microbiol. 1989 Oct;55(10):2723–2725. doi: 10.1128/aem.55.10.2723-2725.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Role of chemical concentration and second carbon sources in acclimation of microbial communities for biodegradation. Appl Environ Microbiol. 1988 Nov;54(11):2803–2807. doi: 10.1128/aem.54.11.2803-2807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Jones S. H., Alexander M. Explanations for the acclimation period preceding the mineralization of organic chemicals in aquatic environments. Appl Environ Microbiol. 1987 Apr;53(4):791–796. doi: 10.1128/aem.53.4.791-796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


