Abstract
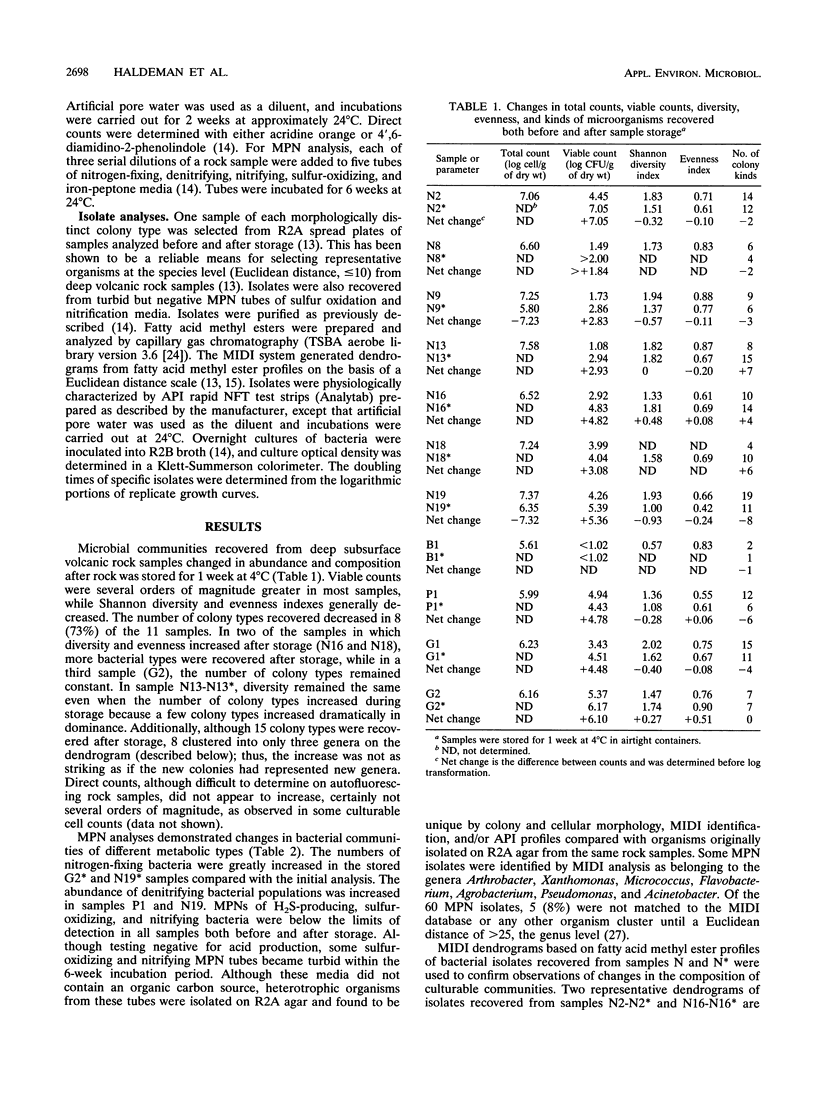
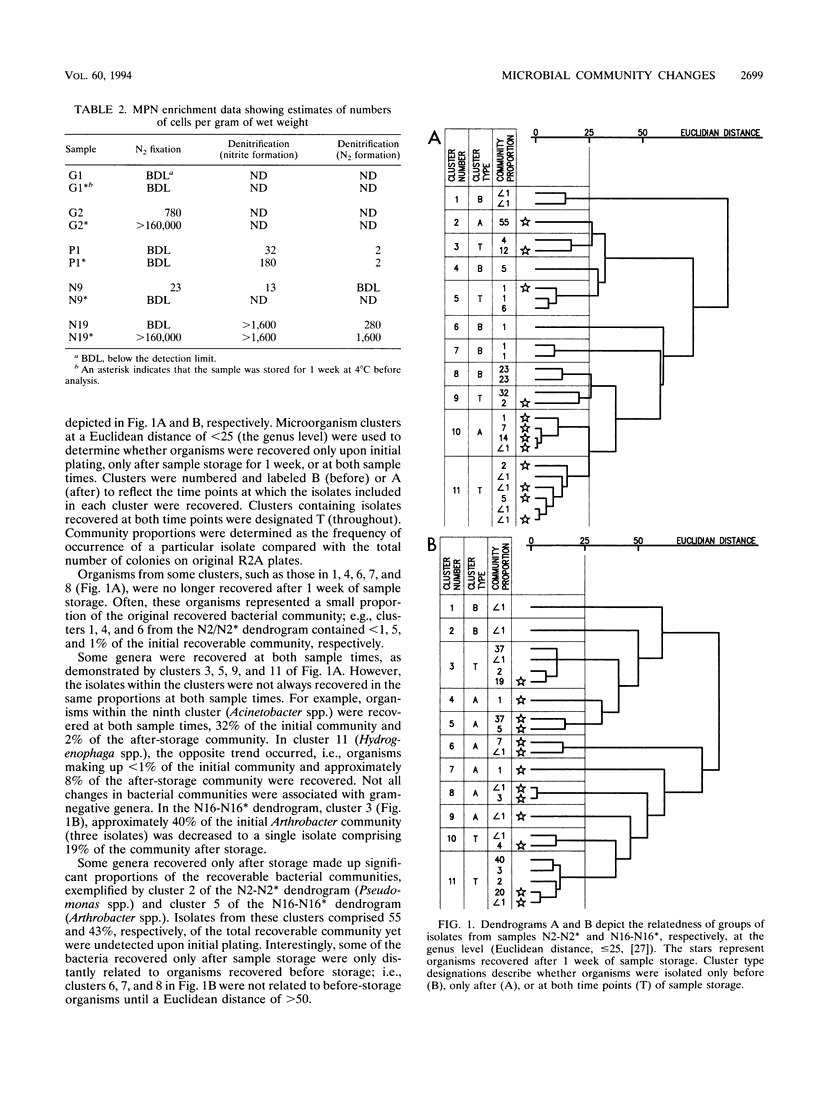
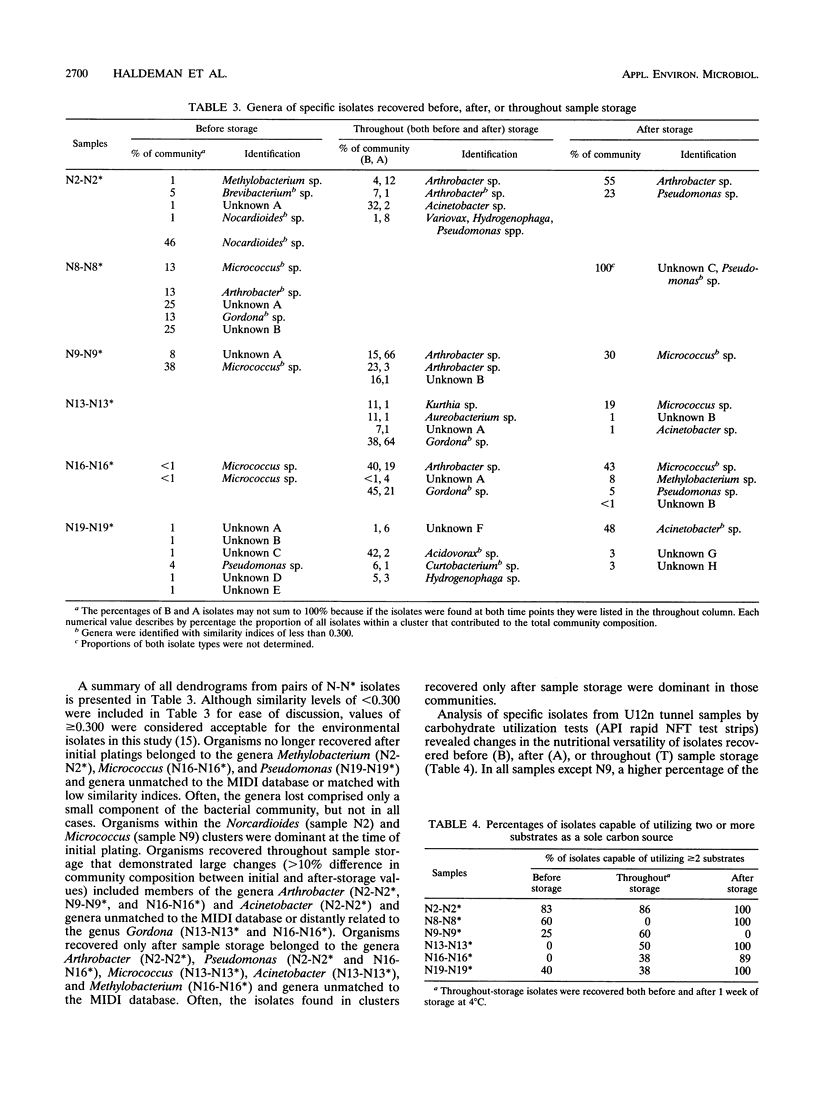
The abundance of viable microorganisms recovered from deep subsurface volcanic rock samples increased after rock perturbation and storage for 1 week at 4°C, while the diversity and evenness of recoverable heterotrophic bacterial communities generally decreased. One sample of each morphologically distinct colony type, recovered both before and after storage of U12n rock samples, was purified and characterized by fatty acid methyl ester (MIDI) and API rapid NFT strips. As determined by MIDI cluster analysis, the composition of the recoverable microbial communities changed with storage of rock samples; some groups of organisms were recovered only before, only after, or at both sample times. In general, the isolates recovered only after storage of rock samples had a greater ability to utilize the carbohydrates included in API test strips and had faster generation times than isolates recovered only on initial plating. The nutritional versatility and faster growth rates of organisms recovered in higher proportions after sample storage provide evidence that some microbial community changes may be due to the proliferation of a few bacterial types. However, because some new genera are recovered only after storage, the possibility also exists that dormant bacterial types are resuscitated during sample perturbation and storage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Haldeman D. L., Ringelberg D., Hall D. H., Russell C. Comparison of Identification Systems for Classification of Bacteria Isolated from Water and Endolithic Habitats within the Deep Subsurface. Appl Environ Microbiol. 1992 Oct;58(10):3367–3373. doi: 10.1128/aem.58.10.3367-3373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Fredrickson J. K., Thomas J. M. Vertical and horizontal variations in the physiological diversity of the aerobic chemoheterotrophic bacterial microflora in deep southeast coastal plain subsurface sediments. Appl Environ Microbiol. 1989 May;55(5):1058–1065. doi: 10.1128/aem.55.5.1058-1065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Influence of environmental stress on enumeration of indicator bacteria from natural waters. Appl Microbiol. 1975 Feb;29(2):186–194. doi: 10.1128/am.29.2.186-194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. J., Xu H. S., Colwell R. R. Viable but nonculturable bacteria in drinking water. Appl Environ Microbiol. 1991 Mar;57(3):875–878. doi: 10.1128/aem.57.3.875-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francisco A., Hall A. J., Schellenberg J. R., Greenwood A. M., Greenwood B. M. The pattern of infant and childhood mortality in Upper River Division, The Gambia. Ann Trop Paediatr. 1993;13(4):345–352. doi: 10.1080/02724936.1993.11747669. [DOI] [PubMed] [Google Scholar]
- Haldeman D. L., Amy P. S. Diversity within a Colony Morphotype: Implications for Ecological Research. Appl Environ Microbiol. 1993 Mar;59(3):933–935. doi: 10.1128/aem.59.3.933-935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. W., Martin W. J. Microwave oven for melting laboratory media. J Clin Microbiol. 1978 Apr;7(4):401–402. doi: 10.1128/jcm.7.4.401-402.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprelyants A. S., Kell D. B. Dormancy in Stationary-Phase Cultures of Micrococcus luteus: Flow Cytometric Analysis of Starvation and Resuscitation. Appl Environ Microbiol. 1993 Oct;59(10):3187–3196. doi: 10.1128/aem.59.10.3187-3196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. G., Vela G. R. True morphology of the Azotobacteraceae-filterable bacteria. Nature. 1981 Feb 12;289(5798):588–590. doi: 10.1038/289588a0. [DOI] [PubMed] [Google Scholar]
- Macdonell M. T., Hood M. A. Isolation and characterization of ultramicrobacteria from a gulf coast estuary. Appl Environ Microbiol. 1982 Mar;43(3):566–571. doi: 10.1128/aem.43.3.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Oliver J. D., Kjelleberg S. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol. 1991 Aug;173(16):5054–5059. doi: 10.1128/jb.173.16.5054-5059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]


