Abstract
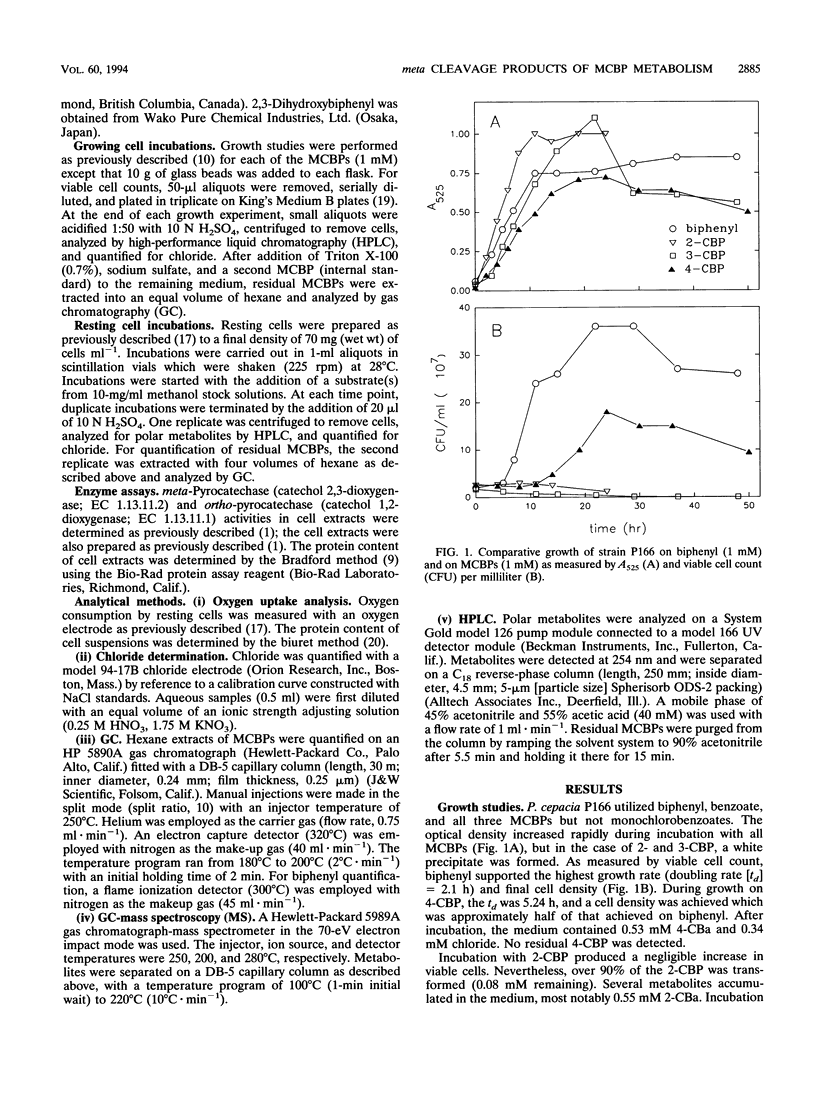
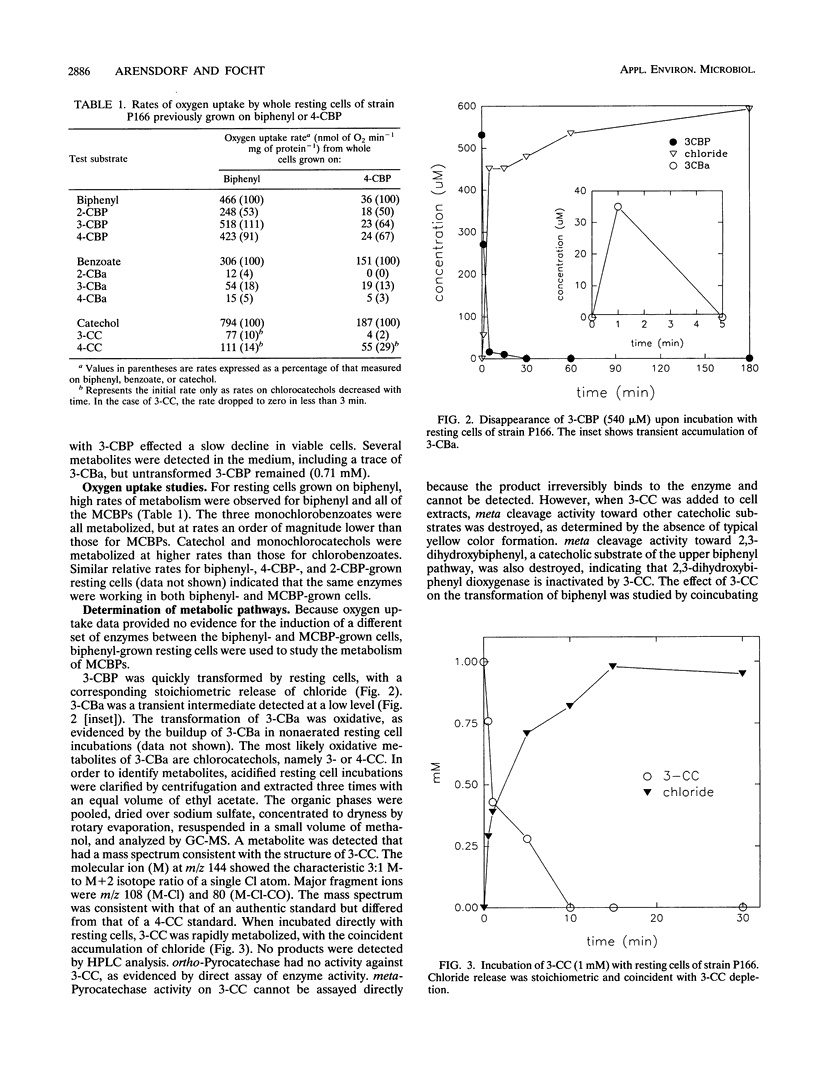
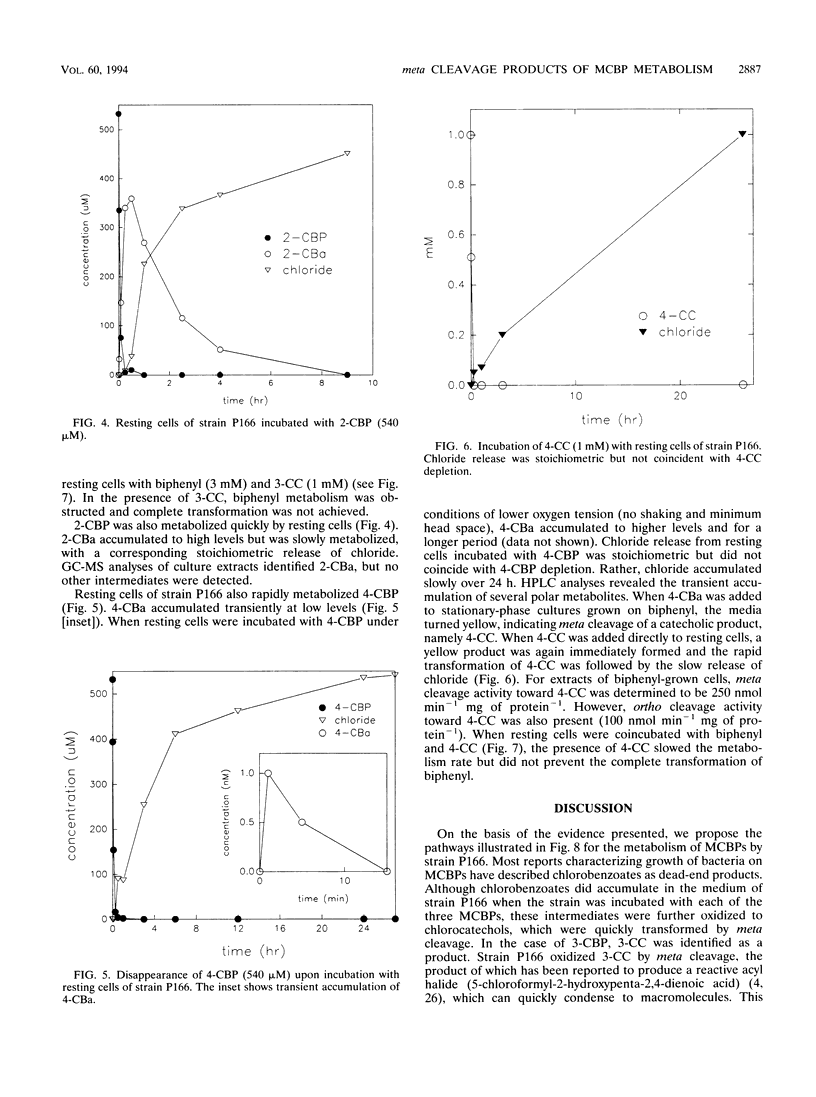
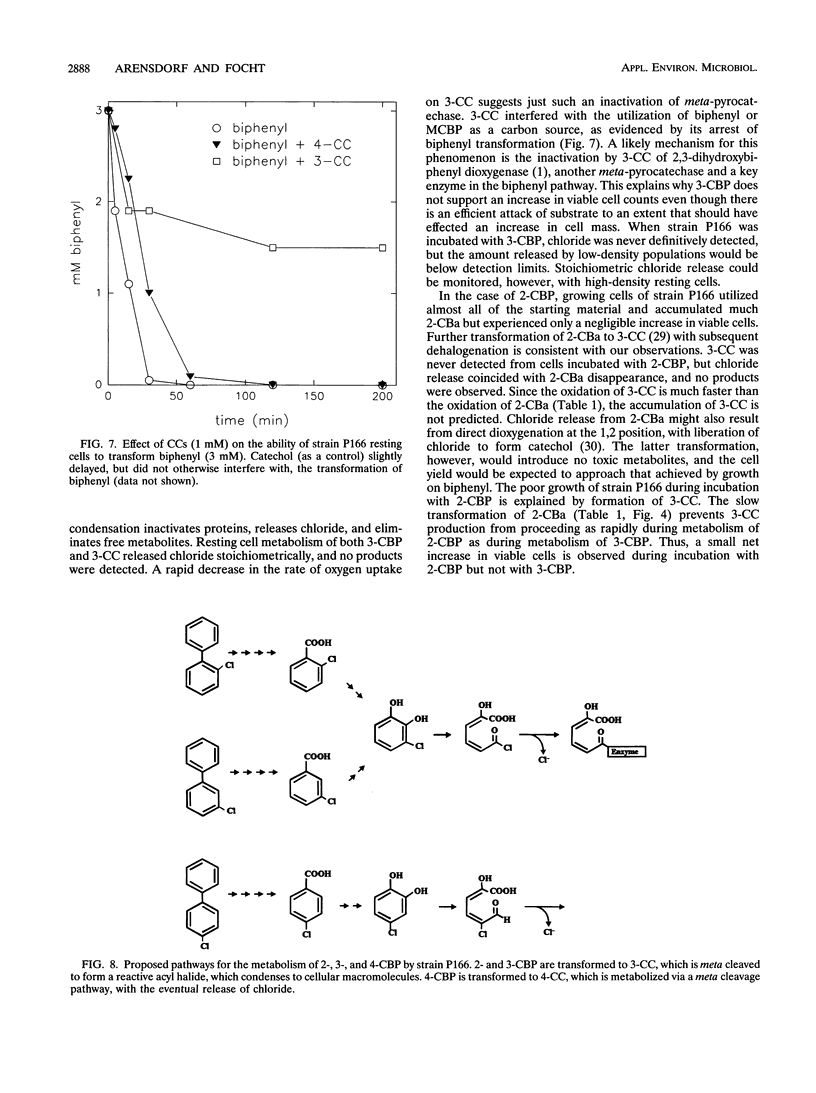
Pseudomonas cepacia P166 was able to metabolize all monochlorobiphenyls to the respective chlorobenzoates. Although they transiently accumulated, the chlorobenzoate degradation intermediates were further metabolized to chlorocatechols, which in turn were meta cleaved. 2- and 3-Chlorobiphenyl both produced 3-chlorocatechol, which was transformed to an acyl halide upon meta cleavage. 3-Chlorocatechol metabolism was toxic to the cells and impeded monochlorobiphenyl metabolism. In the case of 2-chlorobiphenyl, toxicity was manifested as a diminished growth rate, which nevertheless effected rapid substrate utilization. In the case of 3-chlorobiphenyl, which generates 3-chlorocatechol more rapidly than does 2-chlorobiphenyl, toxicity was manifested as a decrease in viable cells during substrate utilization. 4-Chlorobenzoate was transformed to 4-chlorocatechol, which was metabolized by a meta cleavage pathway leading to dehalogenation. Chloride release from 4-chlorocatechol metabolism, however, was slow and did not coincide with rapid 4-chlorocatechol turnover. Growth experiments with strain P166 on monochlorobiphenyls illustrated the difficulties of working with hydrophobic substrates that generate toxic intermediates. Turbidity could not be used to measure the growth of bacteria utilizing monochlorobiphenyls because high turbidities were routinely measured from cultures with very low viable-cell counts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. H., Huang C. M., Higson F. K., Brenner V., Focht D. D. Construction of a 3-chlorobiphenyl-utilizing recombinant from an intergeneric mating. Appl Environ Microbiol. 1992 Feb;58(2):647–654. doi: 10.1128/aem.58.2.647-654.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad D., Massé R., Sylvestre M. Cloning and expression of genes involved in 4-chlorobiphenyl transformation by Pseudomonas testosteroni: homology to polychlorobiphenyl-degrading genes in other bacteria. Gene. 1990 Jan 31;86(1):53–61. doi: 10.1016/0378-1119(90)90113-6. [DOI] [PubMed] [Google Scholar]
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Bartels I., Knackmuss H. J., Reineke W. Suicide Inactivation of Catechol 2,3-Dioxygenase from Pseudomonas putida mt-2 by 3-Halocatechols. Appl Environ Microbiol. 1984 Mar;47(3):500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M. R., Crawford R. L. Novel biotransformations of 4-chlorobiphenyl by a Pseudomonas sp. Appl Environ Microbiol. 1988 Feb;54(2):594–595. doi: 10.1128/aem.54.2.594-595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard D. L., Wagner R. E., Brennan M. J., Haberl M. L., Brown J. F., Jr Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner V., Hernandez B. S., Focht D. D. Variation in chlorobenzoate catabolism by Pseudomonas putida P111 as a consequence of genetic alterations. Appl Environ Microbiol. 1993 Sep;59(9):2790–2794. doi: 10.1128/aem.59.9.2790-2794.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelani D., Sorlini C., Treccani V. The metabolism of biphenyl by Pseudomonas putida. Experientia. 1971 Oct 15;27(10):1173–1174. doi: 10.1007/BF02286908. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Miyazaki T. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J Bacteriol. 1986 May;166(2):392–398. doi: 10.1128/jb.166.2.392-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl Environ Microbiol. 1979 Aug;38(2):301–310. doi: 10.1128/aem.38.2.301-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Hickey W. J., Brenner V., Focht D. D. Mineralization of 2-chloro- and 2,5-dichlorobiphenyl by Pseudomonas sp. strain UCR2. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):175–180. doi: 10.1016/0378-1097(92)90151-d. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Khan A., Walia S. Cloning of bacterial genes specifying degradation of 4-chlorobiphenyl from Pseudomonas putida OU83. Appl Environ Microbiol. 1989 Apr;55(4):798–805. doi: 10.1128/aem.55.4.798-805.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna M., Someda K., Morita K., Yamanouchi Y., Kurimoto T., Kawamura Y., Matsumura H. [Ischemic cerebral symptoms after subarachnoid hemorrhage due to aneurysmal rupture (author's transl)]. No Shinkei Geka. 1978 Jun;6(6):543–548. [PubMed] [Google Scholar]
- Layton A. C., Sanseverino J., Wallace W., Corcoran C., Sayler G. S. Evidence for 4-chlorobenzoic acid dehalogenation mediated by plasmids related to pSS50. Appl Environ Microbiol. 1992 Jan;58(1):399–402. doi: 10.1128/aem.58.1.399-402.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé R., Messier F., Péloquin L., Ayotte C., Sylvestre M. Microbial biodegradation of 4-chlorobiphenyl, a model compound of chlorinated biphenyls. Appl Environ Microbiol. 1984 May;47(5):947–951. doi: 10.1128/aem.47.5.947-951.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello F. J. Cloning and expression in Escherichia coli of Pseudomonas strain LB400 genes encoding polychlorinated biphenyl degradation. J Bacteriol. 1989 Mar;171(3):1725–1732. doi: 10.1128/jb.171.3.1725-1732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M. S., Hooper S. W., Sayler G. S. Plasmid-mediated mineralization of 4-chlorobiphenyl. J Bacteriol. 1985 Sep;163(3):882–889. doi: 10.1128/jb.163.3.882-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondossi M., Sylvestre M., Ahmad D. Effects of chlorobenzoate transformation on the Pseudomonas testosteroni biphenyl and chlorobiphenyl degradation pathway. Appl Environ Microbiol. 1992 Feb;58(2):485–495. doi: 10.1128/aem.58.2.485-495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre M., Mailhiot K., Ahmad D., Massé R. Isolation and preliminary characterization of a 2-chlorobenzoate degrading Pseudomonas. Can J Microbiol. 1989 Apr;35(4):439–443. doi: 10.1139/m89-067. [DOI] [PubMed] [Google Scholar]


