Abstract
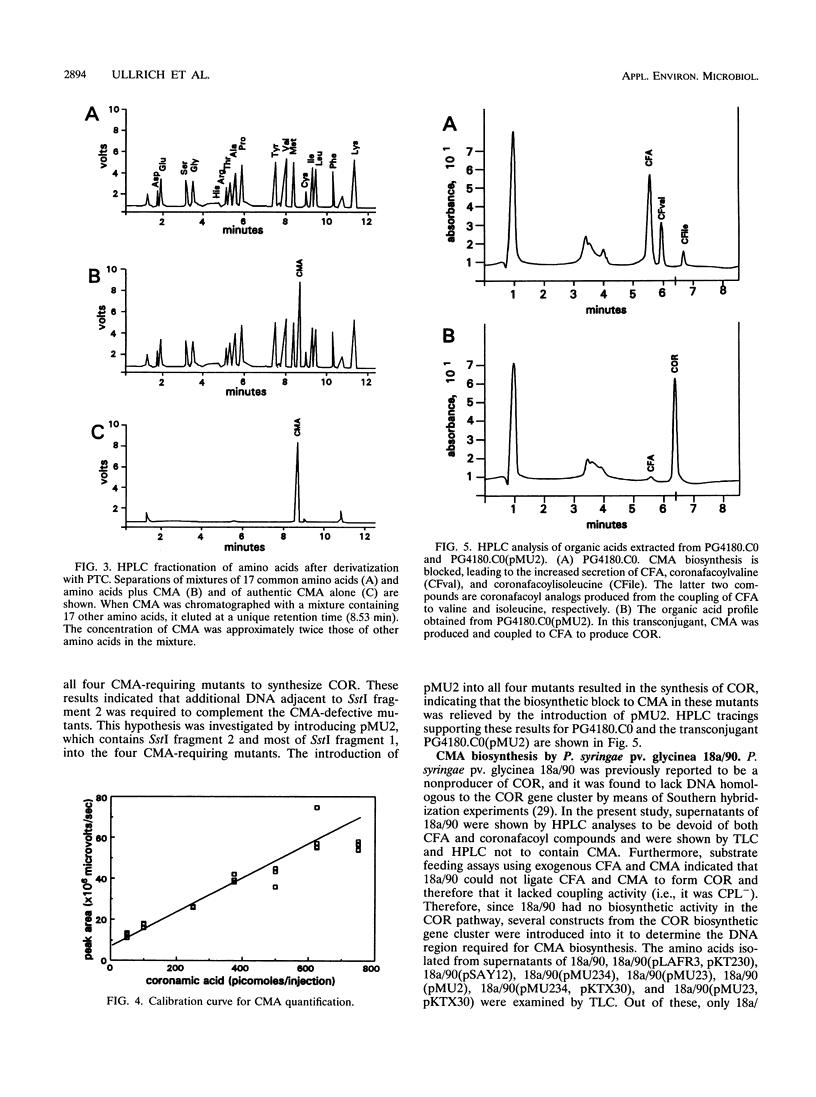
Coronamic acid (CMA; 2-ethyl-1-aminocyclopropane 1-carboxylic acid) is an intermediate in the biosynthesis of coronatine (COR), a chlorosis-inducing phytotoxin produced by Pseudomonas syringae pv. glycinea PG4180. Tn5 mutagenesis and substrate feeding studies were previously used to characterize regions of the COR biosynthetic gene cluster required for synthesis of coronafacic acid and CMA, which are the only two characterized intermediates in the COR biosynthetic pathway. In the present study, additional Tn5 insertions were generated to more precisely define the region required for CMA biosynthesis. A new analytical method for CMA detection which involves derivatization with phenylisothiocyanate and detection by high-performance liquid chromatography (HPLC) was developed. This method was used to analyze and quantify the production of CMA by selected derivatives of P. syringae pv. glycinea which contained mutagenized or cloned regions from the CMA biosynthetic region. pMU2, a clone containing a 6.45-kb insert from the CMA region, genetically complemented mutants which required CMA for COR production. When pMU2 was introduced into P. syringae pv. glycinea 18a/90 (a strain which does not synthesize COR or its intermediates), CMA was not produced, indicating that pMU2 does not contain the complete CMA biosynthetic gene cluster. However, when two plasmid constructs designated pMU234 (12.5 kb) and pKTX30 (3.0 kb) were cointroduced into 18a/90, CMA was detected in culture supernatants by thin-layer chromatography and HPLC. The biological activity of the CMA produced by P. syringae pv. glycinea 18a/90 derivatives was demonstrated by the production of COR in cosynthesis experiments in which 18a/90 transconjugants were cocultivated with CMA-requiring mutants of P. syringae pv. glycinea PG4180. CMA production was also obtained when pMU234 and pKTX30 were cointroduced into P. syringae pv. syringae B1; however, these two constructs did not enable Escherichia coli K-12 to synthesize CMA. The production of CMA in P. syringae strains which lack the COR biosynthetic gene cluster indicates that CMA production can occur independently of coronafacic acid biosynthesis and raises interesting questions regarding the evolutionary origin of the COR biosynthetic pathway.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Baldwin J. E., Abraham E. The biosynthesis of penicillins and cephalosporins. Nat Prod Rep. 1988 Apr;5(2):129–145. doi: 10.1039/np9880500129. [DOI] [PubMed] [Google Scholar]
- Bender C. L., Cooksey D. A. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriol. 1986 Feb;165(2):534–541. doi: 10.1128/jb.165.2.534-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Liyanage H., Palmer D., Ullrich M., Young S., Mitchell R. Characterization of the genes controlling the biosynthesis of the polyketide phytotoxin coronatine including conjugation between coronafacic and coronamic acid. Gene. 1993 Oct 29;133(1):31–38. doi: 10.1016/0378-1119(93)90221-n. [DOI] [PubMed] [Google Scholar]
- Bender C. L., Malvick D. K., Mitchell R. E. Plasmid-mediated production of the phytotoxin coronatine in Pseudomonas syringae pv. tomato. J Bacteriol. 1989 Feb;171(2):807–812. doi: 10.1128/jb.171.2.807-812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Young S. A., Mitchell R. E. Conservation of Plasmid DNA Sequences in Coronatine-Producing Pathovars of Pseudomonas syringae. Appl Environ Microbiol. 1991 Apr;57(4):993–999. doi: 10.1128/aem.57.4.993-999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Ferguson I. B., Mitchell R. E. Stimulation of ethylene production in bean leaf discs by the pseudomonad phytotoxin coronatine. Plant Physiol. 1985 Apr;77(4):969–973. doi: 10.1104/pp.77.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kenyon J. S., Turner J. G. The Stimulation of Ethylene Synthesis in Nicotiana tabacum Leaves by the Phytotoxin Coronatine. Plant Physiol. 1992 Sep;100(1):219–224. doi: 10.1104/pp.100.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. A., Bender C. L. Effects of Environmental and Nutritional Factors on Production of the Polyketide Phytotoxin Coronatine by Pseudomonas syringae pv. Glycinea. Appl Environ Microbiol. 1993 May;59(5):1619–1626. doi: 10.1128/aem.59.5.1619-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salowe S. P., Marsh E. N., Townsend C. A. Purification and characterization of clavaminate synthase from Streptomyces clavuligerus: an unusual oxidative enzyme in natural product biosynthesis. Biochemistry. 1990 Jul 10;29(27):6499–6508. doi: 10.1021/bi00479a023. [DOI] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich M., Bereswill S., Völksch B., Fritsche W., Geider K. Molecular characterization of field isolates of Pseudomonas syringae pv. glycinea differing in coronatine production. J Gen Microbiol. 1993 Aug;139(8):1927–1937. doi: 10.1099/00221287-139-8-1927. [DOI] [PubMed] [Google Scholar]
- Young S. A., Park S. K., Rodgers C., Mitchell R. E., Bender C. L. Physical and functional characterization of the gene cluster encoding the polyketide phytotoxin coronatine in Pseudomonas syringae pv. glycinea. J Bacteriol. 1992 Mar;174(6):1837–1843. doi: 10.1128/jb.174.6.1837-1843.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


