Abstract
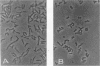
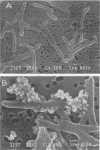
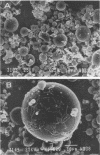
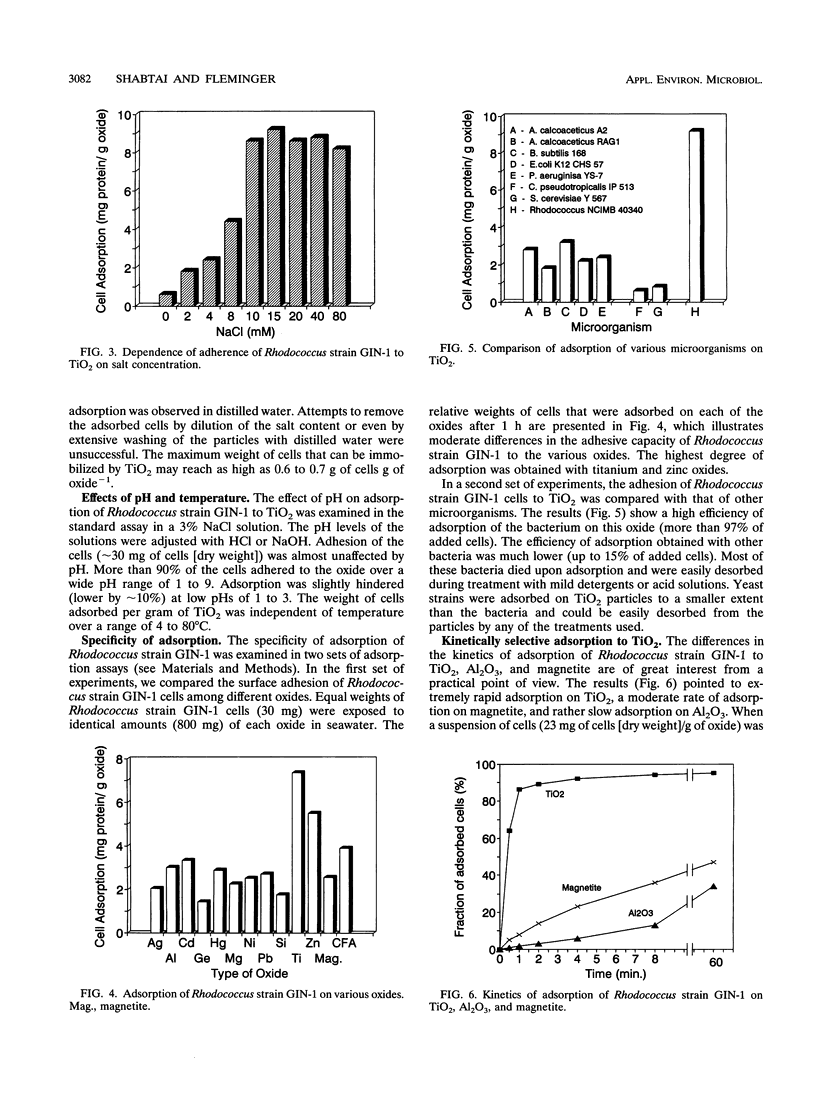
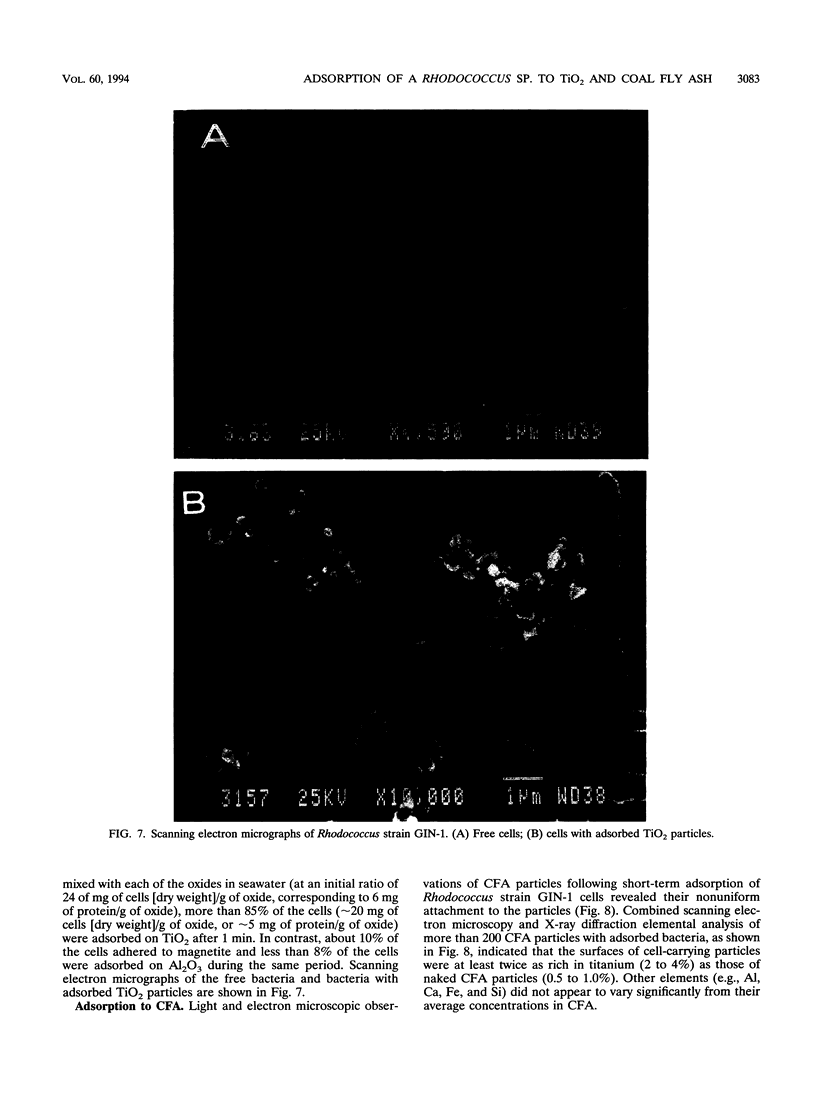
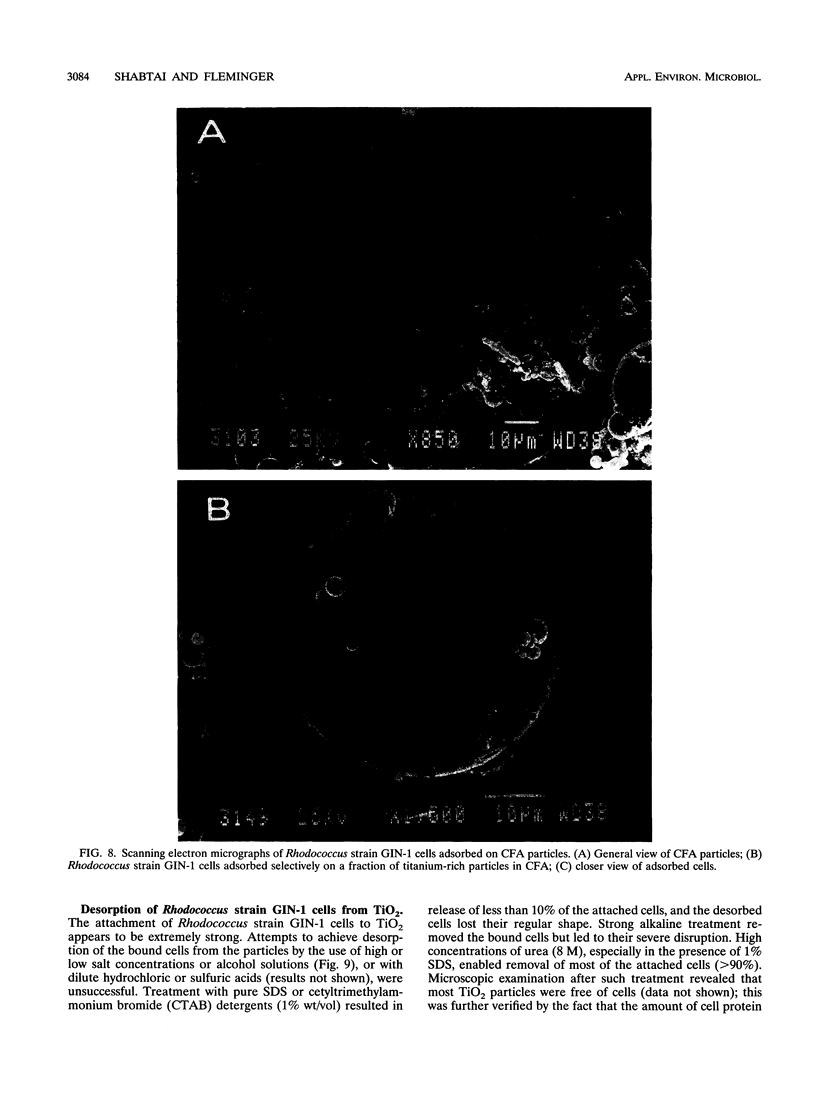
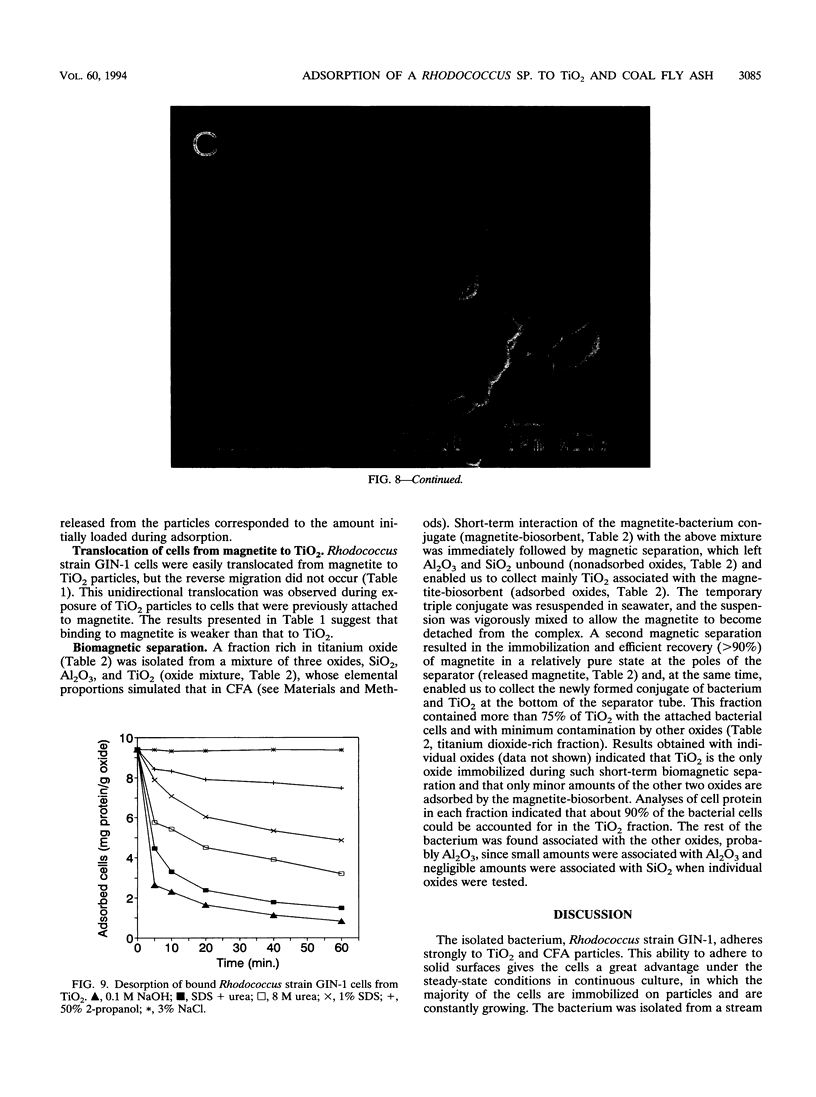
Rhodococcus strain GIN-1 (NCIMB 40340) can be used to enrich and isolate a titanium-rich fraction from coal fly ash. The gram-positive bacterium was isolated by its ability to adhere strongly and rapidly to suspended particles of pure titanium dioxide or coal fly ash. Adsorption depends on the salt concentration and occurs in seawater. Lowering of the salt concentration or washing of particles with pure water did not, however, cause desorption of the bacteria from TiO2 particles; this was achieved by strong alkaline treatment or combined treatment with sodium dodecyl sulfate and urea but not with dilute acids, alcohols, or cationic or nonionic detergents. The bacterium exhibits higher affinity towards oxides of Ti and Zn than to other oxides with similar distribution of particle size. Moreover, it adheres much faster to TiO2 than to magnetite (Fe3O4) or Al2O3. After about 1 min, more than 85% of the cells were adsorbed on TiO2, compared with adsorption of only 10 and 8% to magnetite and Al2O3, respectively. Adsorption of the bacteria on TiO2 occurs over a pH range of 1.0 to 9.0 and at temperatures from 4 to over 80°C. Scanning electron microscopy combined with X-ray analysis revealed preferential adherence of the bacterium to coal ash particles richer in Ti. Stronger adhesion to TiO2 was also demonstrated in the translocation of bacteria, preadsorbed on magnetite, to TiO2 particles. The temporary co-adhesion to magnetite and TiO2 was exploited for the design of a prototype biomagnetic separation process in which bacterial cells serve as an adhesive mediator between magnetite and TiO2 particles in a mixture of Al, Si, and Ti oxides that simulates their proportion in the ash.
Full text
PDF

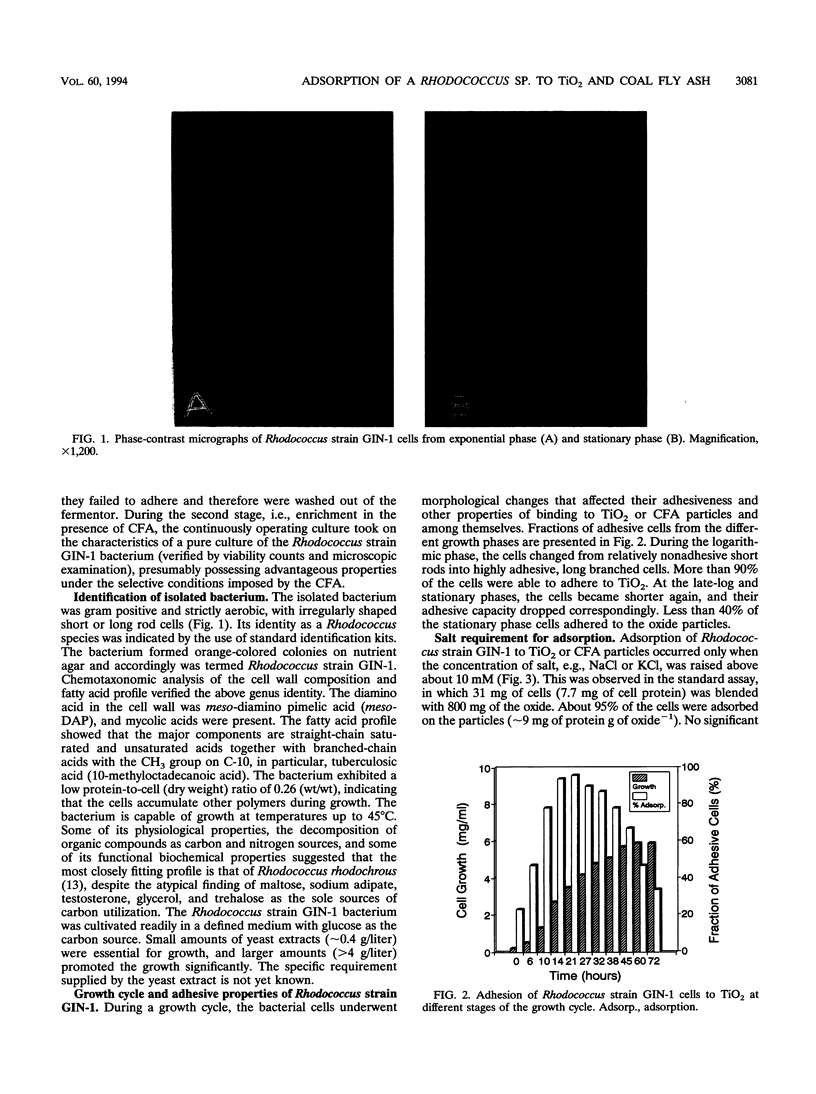



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendinger B., Rijnaarts H. H., Altendorf K., Zehnder A. J. Physicochemical cell surface and adhesive properties of coryneform bacteria related to the presence and chain length of mycolic acids. Appl Environ Microbiol. 1993 Nov;59(11):3973–3977. doi: 10.1128/aem.59.11.3973-3977.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Fayerweather W. E. Epidemiologic study of workers exposed to titanium dioxide. J Occup Med. 1988 Dec;30(12):937–942. doi: 10.1097/00043764-198812000-00011. [DOI] [PubMed] [Google Scholar]
- Connors K. A., Jozwiakowski M. J. Studies on adsorptiochromism. I: Binding of adsorptiochromic spiropyrans to some pharmaceutically useful solids. J Pharm Sci. 1987 Dec;76(12):892–897. doi: 10.1002/jps.2600761210. [DOI] [PubMed] [Google Scholar]
- Joshi R. I., Eley A. The in-vitro effect of a titanium implant on oral microflora: comparison with other metallic compounds. J Med Microbiol. 1988 Oct;27(2):105–107. doi: 10.1099/00222615-27-2-105. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. P., Henry N. W., 3rd, Trochimowicz H. J., Reinhardt C. F. Pulmonary response to impaired lung clearance in rats following excessive TiO2 dust deposition. Environ Res. 1986 Oct;41(1):144–167. doi: 10.1016/s0013-9351(86)80177-3. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]