Abstract
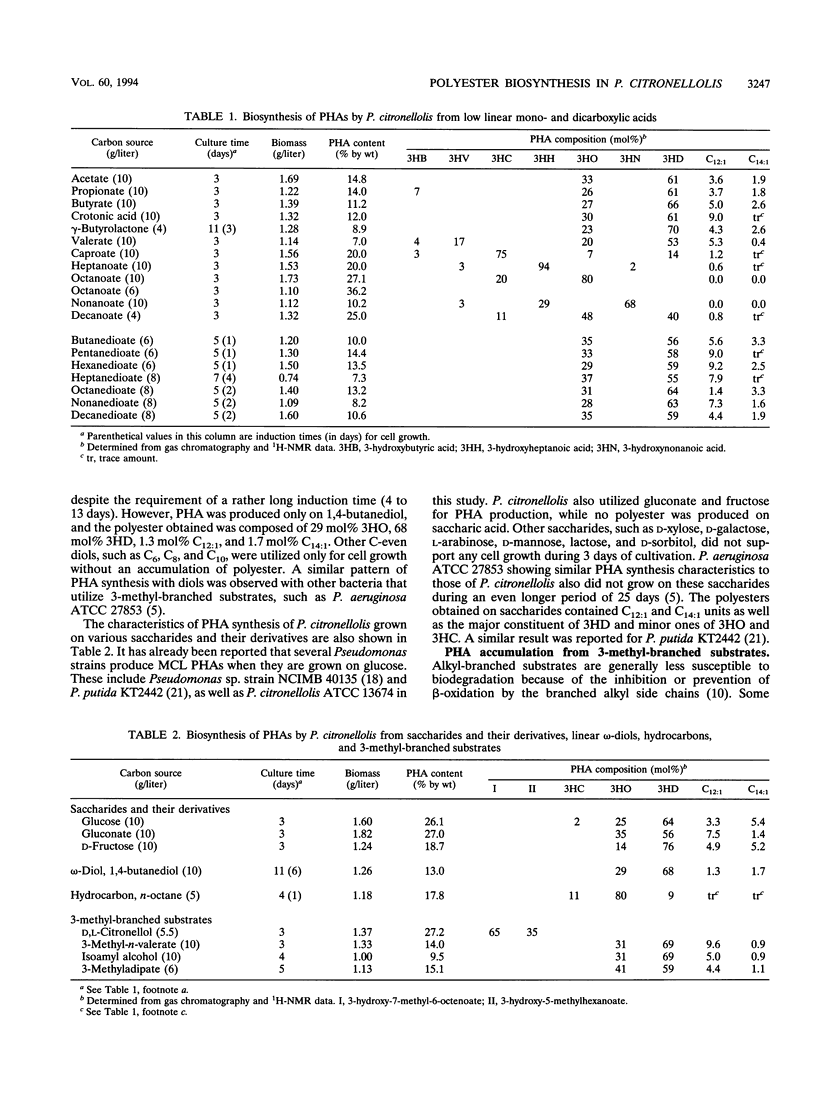
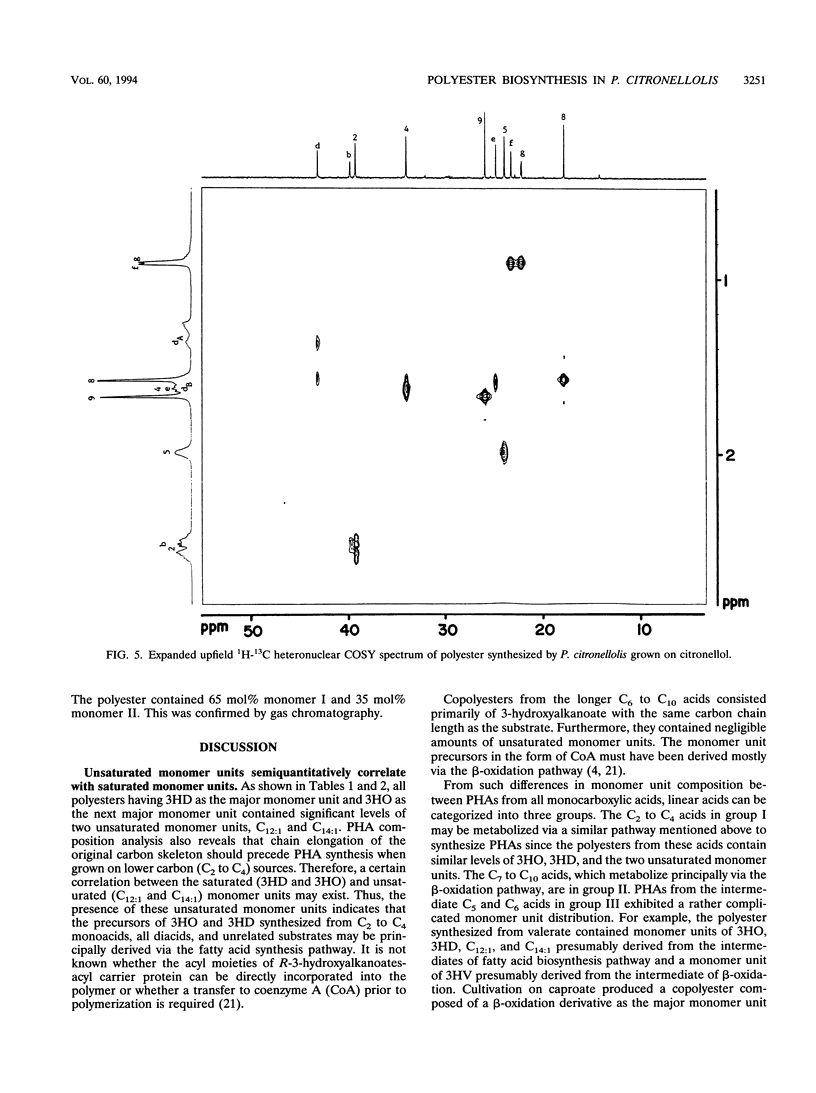
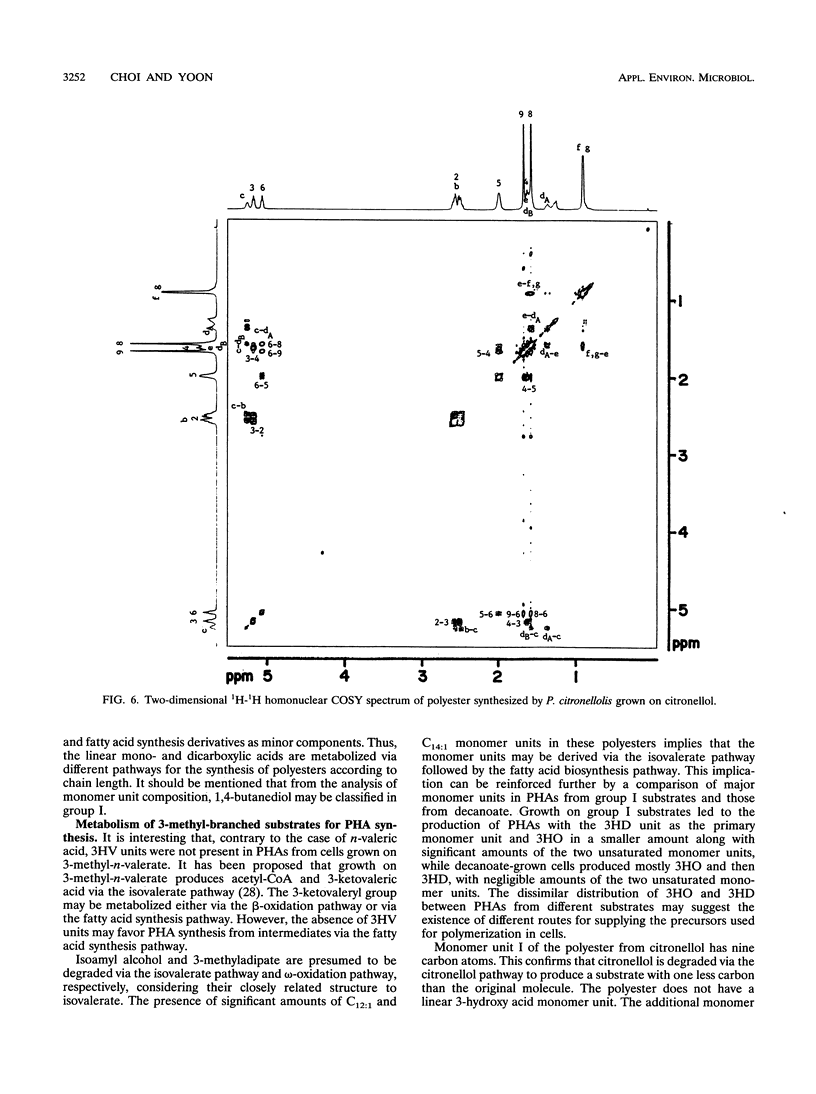
Forty-two different carbon sources were tested for the polyester synthesis of a citronellol-utilizing bacterium, Pseudomonas citronellolis (ATCC 13674). These included linear C2 to C10 monocarboxylic acids, C3 to C10 dicarboxylic acids, saccharides, α,ω-diols, hydrocarbons, and 3-methyl-branched substrates such as 3,7-dimethyl-6-octen-1-ol (citronellol), 3-methyl-n-valerate, 3-methyl-1-butanol, and 3-methyladipate. Isolated polymers were characterized by gas chromatography, infrared spectroscopy, 1H- or 13C-nuclear magnetic resonance spectroscopy, 1H-13C heteronuclear correlation spectroscopy (1H-13C COSY), 1H-1H homonuclear COSY, and differential scanning calorimetry. Polyesters from nine monocarboxylic acids and two related carbon sources could be metabolically divided into three groups. The first group of C2 to C4 carbon sources resulted in copolyesters composed of 61 to 70 mol% 3-hydroxydecanoate, 23 to 33 mol% 3-hydroxyoctanoate, 3.6 to 9.0 mol% 3-hydroxy-5-cis-dodecenoate, and 1.8 to 2.6 mol% 3-hydroxy-7-cis-tetradecenoate. Carbon sources in group II (C7 to C10) produced copolyesters composed of 3-hydroxyacid monomer units with the same number of carbon atoms as the substrate (major constituent) and monomer units with either two less or two more carbons. Negligible amounts of 3-hydroxy-5-cis-dodecenoate and 3-hydroxy-7-cis-tetradecenoate were detected in copolyesters from this group. Copolyesters from group III (C5 and C6) had a monomer unit distribution that could be said to be between those of groups I and II. In addition, a novel copolyester, poly(3-hydroxy-7-methyl-6-octenoate-co-3-hydroxy-5-methylhexanoate), was synthesized when grown on citronellol. The 1H-13C heteronuclear COSY spectrum for monomer unit II revealed that both methylene and isopropyl groups, proximately connected in series to a single chiral center, had magnetically diastereotopic natures.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Dawes E. A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990 Dec;54(4):450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J. L., Ramsay B. A., Ramsay J. A., Chavarie C. Biosynthesis of Poly-beta-Hydroxyalkanoates from Pentoses by Pseudomonas pseudoflava. Appl Environ Microbiol. 1990 Oct;56(10):3133–3138. doi: 10.1128/aem.56.10.3133-3138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Knee E. J., Jr, Fuller R. C., Gross R. A., Lenz R. W. Ability of the phototrophic bacterium Rhodospirillum rubrum to produce various poly (beta-hydroxyalkanoates): potential sources for biodegradable polyesters. Int J Biol Macromol. 1989 Feb;11(1):49–55. doi: 10.1016/0141-8130(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Fall R. R., Brown J. L., Schaeffer T. L. Enzyme recruitment allows the biodegradation of recalcitrant branched hydrocarbons by Pseudomonas citronellolis. Appl Environ Microbiol. 1979 Oct;38(4):715–722. doi: 10.1128/aem.38.4.715-722.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche K., Lenz R. W., Fuller R. C. Bacterial polyesters containing branched poly(beta-hydroxyalkanoate) units. Int J Biol Macromol. 1990 Apr;12(2):92–101. doi: 10.1016/0141-8130(90)90059-j. [DOI] [PubMed] [Google Scholar]
- Fritzsche K., Lenz R. W., Fuller R. C. Production of unsaturated polyesters by Pseudomonas oleovorans. Int J Biol Macromol. 1990 Apr;12(2):85–91. doi: 10.1016/0141-8130(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Haywood G. W., Anderson A. J., Ewing D. F., Dawes E. A. Accumulation of a Polyhydroxyalkanoate Containing Primarily 3-Hydroxydecanoate from Simple Carbohydrate Substrates by Pseudomonas sp. Strain NCIMB 40135. Appl Environ Microbiol. 1990 Nov;56(11):3354–3359. doi: 10.1128/aem.56.11.3354-3359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood G. W., Anderson A. J., Williams D. R., Dawes E. A., Ewing D. F. Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus sp. NCIMB 40126. Int J Biol Macromol. 1991 Apr;13(2):83–88. doi: 10.1016/0141-8130(91)90053-w. [DOI] [PubMed] [Google Scholar]
- Huijberts G. N., Eggink G., de Waard P., Huisman G. W., Witholt B. Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl Environ Microbiol. 1992 Feb;58(2):536–544. doi: 10.1128/aem.58.2.536-544.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman G. W., Wonink E., Meima R., Kazemier B., Terpstra P., Witholt B. Metabolism of poly(3-hydroxyalkanoates) (PHAs) by Pseudomonas oleovorans. Identification and sequences of genes and function of the encoded proteins in the synthesis and degradation of PHA. J Biol Chem. 1991 Feb 5;266(4):2191–2198. [PubMed] [Google Scholar]
- Huisman G. W., de Leeuw O., Eggink G., Witholt B. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol. 1989 Aug;55(8):1949–1954. doi: 10.1128/aem.55.8.1949-1954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lageveen R. G., Huisman G. W., Preusting H., Ketelaar P., Eggink G., Witholt B. Formation of Polyesters by Pseudomonas oleovorans: Effect of Substrates on Formation and Composition of Poly-(R)-3-Hydroxyalkanoates and Poly-(R)-3-Hydroxyalkenoates. Appl Environ Microbiol. 1988 Dec;54(12):2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waard P., van der Wal H., Huijberts G. N., Eggink G. Heteronuclear NMR analysis of unsaturated fatty acids in poly(3-hydroxyalkanoates). Study of beta-oxidation in Pseudomonas putida. J Biol Chem. 1993 Jan 5;268(1):315–319. [PubMed] [Google Scholar]


