Abstract
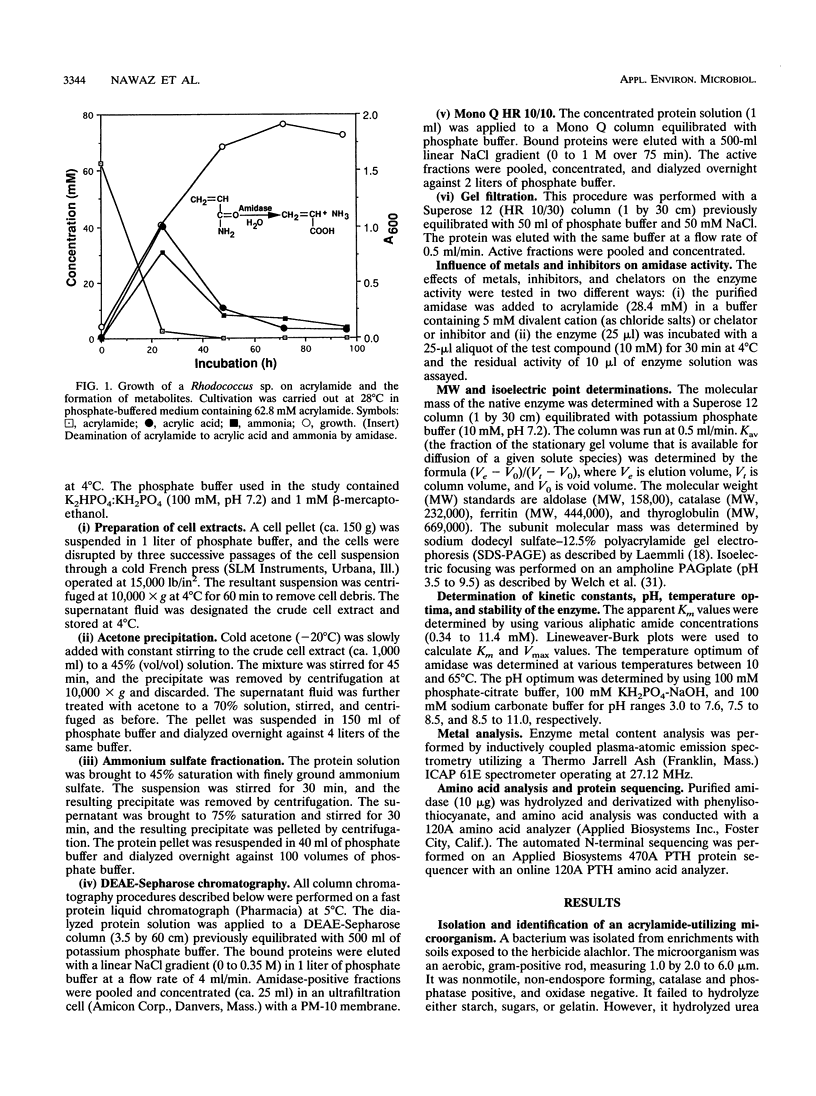
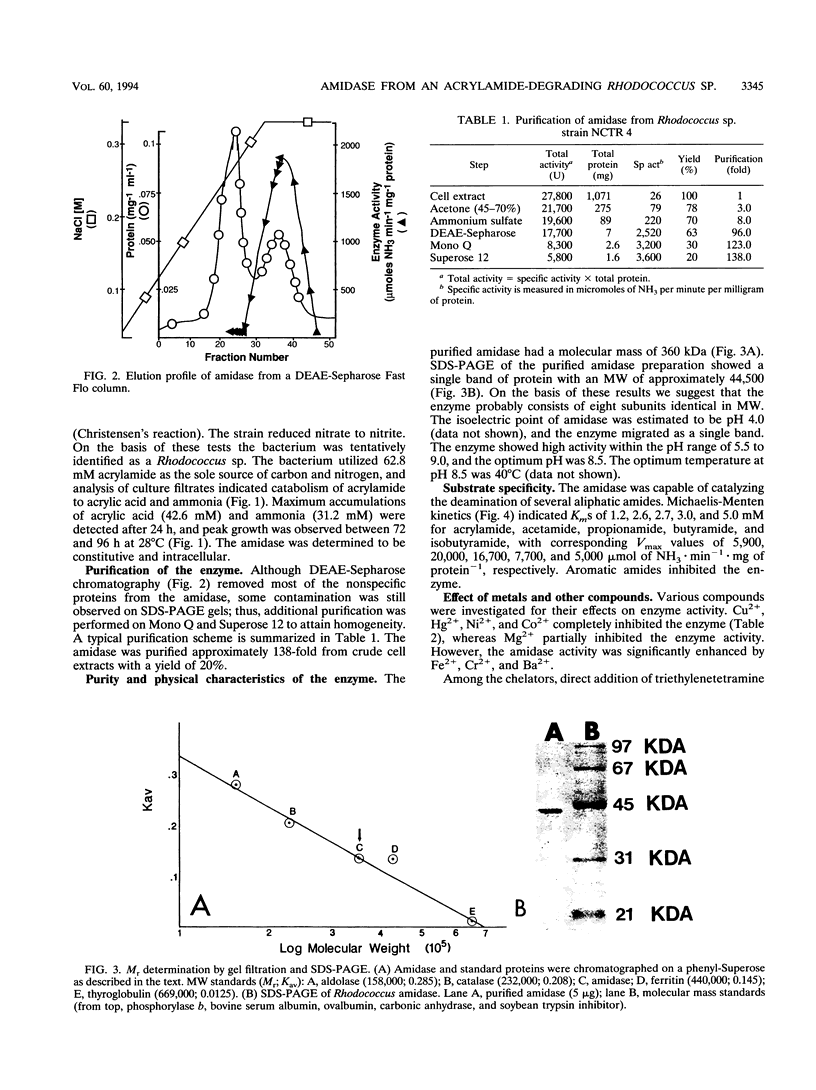
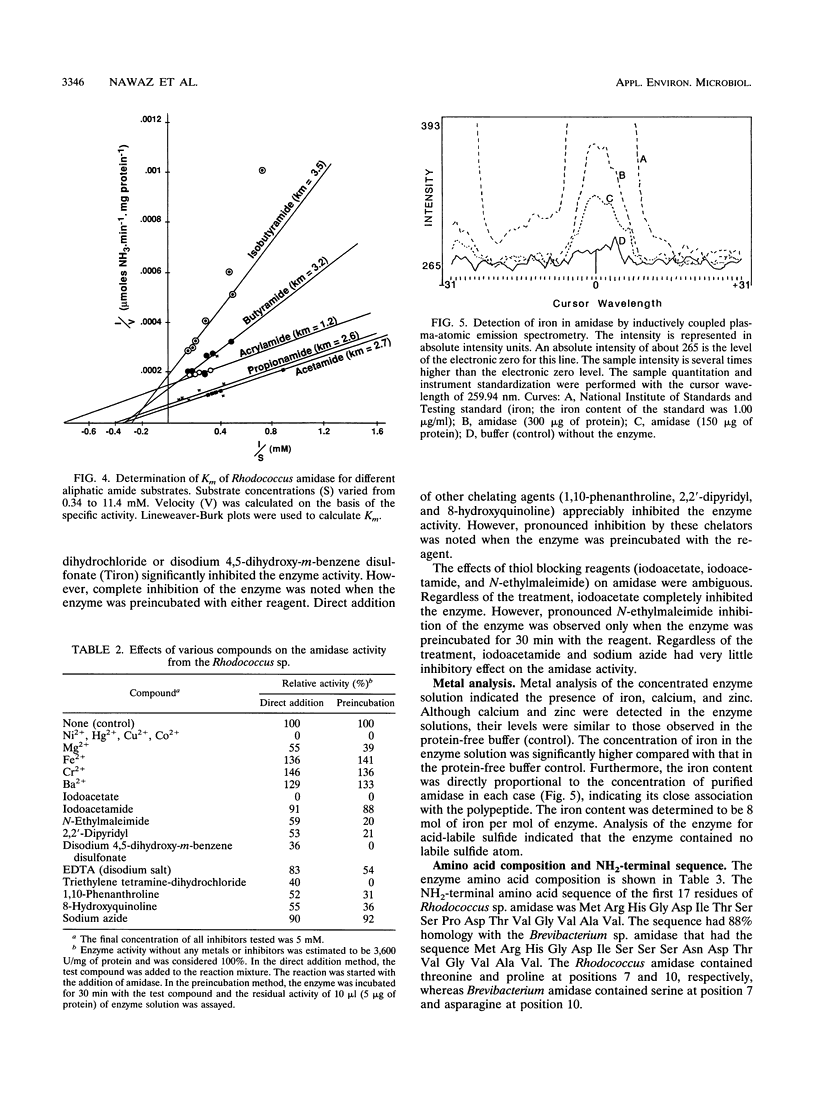
A constitutively expressed aliphatic amidase from a Rhodococcus sp. catalyzing acrylamide deamination was purified to electrophoretic homogeneity. The molecular weight of the native enzyme was estimated to be 360,000. Upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the purified preparation yielded a homogeneous protein band having an apparent molecular weight of about 44,500. The amidase had pH and temperature optima of 8.5 and 40 degrees C, respectively, and its isoelectric point was pH 4.0. The amidase had apparent K(m) values of 1.2, 2.6, 3.0, 2.7, and 5.0 mM for acrylamide, acetamide, butyramide, propionamide, and isobutyramide, respectively. Inductively coupled plasma-atomic emission spectometry analysis indicated that the enzyme contains 8 mol of iron per mol of the native enzyme. No labile sulfide was detected. The amidase activity was enhanced by, but not dependent on Fe(2+), Ba(2+), and Cr(2+). However, the enzyme activity was partially inhibited by Mg(2+) and totally inhibited in the presence of Ni(2+), Hg(2+), Cu(2+), Co(2+), specific iron chelators, and thiol blocking reagents. The NH2-terminal sequence of the first 18 amino acids displayed 88% homology to the aliphatic amidase of Brevibacterium sp. strain R312.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt J., Krisch K. Isolation of an inducible amidase from Pseudomonas acidovorans AE1. J Gen Microbiol. 1975 Apr;87(2):260–272. doi: 10.1099/00221287-87-2-260. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Brown P. R., Clarke P. H. Butyramide-utilizing mutants of Pseudomonas aeruginosa 8602 which produce an amidase with altered substrate specificity. J Gen Microbiol. 1969 Aug;57(2):273–285. doi: 10.1099/00221287-57-2-273. [DOI] [PubMed] [Google Scholar]
- Cavins J. F., Friedman M. Specific modification of protein sulfhydryl groups with alpha,beta-unsaturated compounds. J Biol Chem. 1968 Jun 25;243(12):3357–3360. [PubMed] [Google Scholar]
- Chapatwala K. D., Nawaz M. S., Richardson J. D., Wolfram J. H. Isolation and characterization of acetonitrile utilizing bacteria. J Ind Microbiol. 1990 Apr-May;5(2-3):65–70. doi: 10.1007/BF01573854. [DOI] [PubMed] [Google Scholar]
- Dearfield K. L., Abernathy C. O., Ottley M. S., Brantner J. H., Hayes P. F. Acrylamide: its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutat Res. 1988 Jan;195(1):45–77. doi: 10.1016/0165-1110(88)90015-2. [DOI] [PubMed] [Google Scholar]
- DiGeronimo M. J., Antoine A. D. Metabolism of acetonitrile and propionitrile by Nocardia rhodochrous LL100-21. Appl Environ Microbiol. 1976 Jun;31(6):900–906. doi: 10.1128/aem.31.6.900-906.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt G., Wallnöfer P. R., Plapp R. Purification and properties of an aryl acylamidase of Bacillus sphaericus, catalyzing the hydrolysis of various phenylamide herbicides and fungicides. Appl Microbiol. 1973 Nov;26(5):709–718. doi: 10.1128/am.26.5.709-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. J., Wilson J. V. Degradation and hydrolysis of amides by Corynebacterium pseudodiphtheriticum NCIB 10803. Microbios. 1973 Jun-Aug;8(29):15–22. [PubMed] [Google Scholar]
- Kaplan A. The determination of urea, ammonia, and urease. Methods Biochem Anal. 1969;17:311–324. doi: 10.1002/9780470110355.ch7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nawaz M. S., Chapatwala K. D. Simultaneous degradation of acetonitrile and biphenyl by Pseudomonas aeruginosa. Can J Microbiol. 1991 Jun;37(6):411–418. doi: 10.1139/m91-067. [DOI] [PubMed] [Google Scholar]
- Nawaz M. S., Chapatwala K. D., Wolfram J. H. Degradation of Acetonitrile by Pseudomonas putida. Appl Environ Microbiol. 1989 Sep;55(9):2267–2274. doi: 10.1128/aem.55.9.2267-2274.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M. S., Franklin W., Campbell W. L., Heinze T. M., Cerniglia C. E. Metabolism of acrylonitrile by Klebsiella pneumoniae. Arch Microbiol. 1991;156(3):231–238. doi: 10.1007/BF00249120. [DOI] [PubMed] [Google Scholar]
- Nawaz M. S., Franklin W., Cerniglia C. E. Degradation of acrylamide by immobilized cells of a Pseudomonas sp. and Xanthomonas maltophilia. Can J Microbiol. 1993 Feb;39(2):207–212. doi: 10.1139/m93-029. [DOI] [PubMed] [Google Scholar]
- Neuhäuser-Klaus A., Schmahl W. Mutagenic and teratogenic effects of acrylamide in the mammalian spot test. Mutat Res. 1989 Jul;226(3):157–162. doi: 10.1016/0165-7992(89)90013-4. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Murphy M. J., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. I. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem. 1973 Jan 10;248(1):251–264. [PubMed] [Google Scholar]
- Soubrier F., Lévy-Schil S., Mayaux J. F., Pétré D., Arnaud A., Crouzet J. Cloning and primary structure of the wide-spectrum amidase from Brevibacterium sp. R312: high homology to the amiE product from Pseudomonas aeruginosa. Gene. 1992 Jul 1;116(1):99–104. doi: 10.1016/0378-1119(92)90635-3. [DOI] [PubMed] [Google Scholar]
- Vaughan P. A., Hall G. F., Best D. J. Aryl acylamidase from Rhodococcus erythropolis NCIB 12273. Appl Microbiol Biotechnol. 1990 Oct;34(1):42–46. doi: 10.1007/BF00170921. [DOI] [PubMed] [Google Scholar]
- Welch S. G., Swindlehurst C. A., McGregor I. A., Williams K. Isoelectric focusing of human red cell phosphoglucomutase: the distribution of variant phenotypes in a village population from the Gambia, West Africa. Hum Genet. 1978 Sep 19;43(3):307–313. doi: 10.1007/BF00278838. [DOI] [PubMed] [Google Scholar]



