Abstract
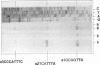
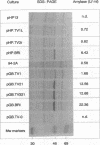
We isolated the gene amyE(TV1) from Thermoactinomyces vulgaris 94-2A encoding a nonglucogenic alpha-amylase (AmyTV1). A chromosomal DNA fragment of 2,247 bp contained an open reading frame of 483 codons, which was expressed in Escherichia coli and Bacillus subtilis. The deduced amino acid sequence of the AmyTV1 protein was confirmed by sequencing of several peptides derived from the enzyme isolated from a T. vulgaris 94-2A culture. The amino acid sequence was aligned with several known alpha-amylase sequences. We found 83% homology with the 48-kDa alpha-amylase part of the Bacillus polymyxa beta-alpha-amylase polyprotein and 50% homology with Taka amylase A of Aspergillus oryzae but only 45% homology with another T. vulgaris amylase (neopullulanase, TVA II) recently cloned from strain R-47. The putative promoter region was characterized with primer extension and deletion experiments and by expression studies with B. subtilis. Multiple promoter sites (P3, P2, and P1) were found; P1 alone drives about 1/10 of the AmyTV1 expression directed by the native tandem configuration P3P2P1. The expression levels in B. subtilis could be enhanced by fusion of the amyE(TV1) coding region to the promoter of the Bacillus amyloliquefaciens alpha-amylase gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke D., Gilmore M. S., Ferretti J. J. Plasmid pGB301, a new multiple resistance streptococcal cloning vehicle and its use in cloning of a gentamicin/kanamycin resistance determinant. Mol Gen Genet. 1981;182(3):414–421. doi: 10.1007/BF00293929. [DOI] [PubMed] [Google Scholar]
- Behnke U., Kleine R., Ludewig M., Ruttloff H. Charakterisierung einer Protease aus Thermoactinomyces vulgaris (Thermitase). 3. Substratspezifität und einige Eigenschaften der teilgereinigten Thermitase. Acta Biol Med Ger. 1978;37(8):1205–1214. [PubMed] [Google Scholar]
- Betzel C., Teplyakov A. V., Harutyunyan E. H., Saenger W., Wilson K. S. Thermitase and proteinase K: a comparison of the refined three-dimensional structures of the native enzymes. Protein Eng. 1990 Jan;3(3):161–172. doi: 10.1093/protein/3.3.161. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- DEMAIN A. L. Minimal media for quantitative studies with Bacillus subtilis. J Bacteriol. 1958 May;75(5):517–522. doi: 10.1128/jb.75.5.517-522.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R. H., Wang L. F. Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev. 1986 Sep;50(3):227–243. doi: 10.1128/mr.50.3.227-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Frömmel C., Hausdorf G., Höhne W. E., Behnke U., Ruttloff H. Charakterisierung einer Protease aus Thermoactinomyces vulgaris (Thermitase). 2. Einschritt-Feinreinigung und proteinchemische Charakterisierung. Acta Biol Med Ger. 1978;37(8):1193–1204. [PubMed] [Google Scholar]
- Frömmel C., Höhne W. E. Influence of calcium binding on the thermal stability of 'thermitase', a serine protease from Thermoactinomyces vulgaris. Biochim Biophys Acta. 1981 Aug 28;670(1):25–31. doi: 10.1016/0005-2795(81)90044-1. [DOI] [PubMed] [Google Scholar]
- Furusato T., Takano J., Jigami Y., Tanaka H., Yamane K. Two tandemly located promoters, artificially constructed, are active in a Bacillus subtilis alpha-amylase secretion vector. J Biochem. 1986 Apr;99(4):1181–1190. doi: 10.1093/oxfordjournals.jbchem.a135581. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Rabinowitz J. C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for "extended" promoter elements in gram-positive organisms. J Biol Chem. 1986 Aug 25;261(24):11409–11415. [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haima P., Bron S., Venema G. The effect of restriction on shotgun cloning and plasmid stability in Bacillus subtilis Marburg. Mol Gen Genet. 1987 Sep;209(2):335–342. doi: 10.1007/BF00329663. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heese O., Hansen G., Höhne W. E., Körner D. Eine thermostabile alpha-Amylase aus Thermoactinomyces vulgaris: Reinigung und Charakterisierung. Biomed Biochim Acta. 1991;50(3):225–232. [PubMed] [Google Scholar]
- Hoshiko S., Makabe O., Nojiri C., Katsumata K., Satoh E., Nagaoka K. Molecular cloning and characterization of the Streptomyces hygroscopicus alpha-amylase gene. J Bacteriol. 1987 Mar;169(3):1029–1036. doi: 10.1128/jb.169.3.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen H. M., MacGregor E. A., Sierks M. R., Svensson B. Comparison of the domain-level organization of starch hydrolases and related enzymes. Biochem J. 1991 Nov 15;280(Pt 1):51–55. doi: 10.1042/bj2800051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine R. Properties of thermitase, a thermostable serine protease from Thermoactinomyces vulgaris. Acta Biol Med Ger. 1982;41(1):89–102. [PubMed] [Google Scholar]
- Klingenberg P., Zickler F., Leuchtenberger A., Ruttloff H. Gewinnung und Charakterisierung von Proteasen aus Thermoactinomyces vulgaris II. Selektion von leistungsfähigen Stämmen. Z Allg Mikrobiol. 1979;19(1):17–25. doi: 10.1002/jobm.3630190104. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Sano T., Ii K., Hizawa K., Yamanoi A., Otsuka T. Mixed connective tissue disease with fatal pulmonary hypertension. Acta Pathol Jpn. 1982 Nov;32(6):1121–1129. doi: 10.1111/j.1440-1827.1982.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Kuriki T., Okada S., Imanaka T. New type of pullulanase from Bacillus stearothermophilus and molecular cloning and expression of the gene in Bacillus subtilis. J Bacteriol. 1988 Apr;170(4):1554–1559. doi: 10.1128/jb.170.4.1554-1559.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Ulmanen I., Palva I. In vivo transcription initiation and termination sites of an alpha-amylase gene from Bacillus amyloliquefaciens cloned in Bacillus subtilis. Gene. 1984 Oct;30(1-3):11–16. doi: 10.1016/0378-1119(84)90099-4. [DOI] [PubMed] [Google Scholar]
- MacGregor E. A. Alpha-amylase structure and activity. J Protein Chem. 1988 Aug;7(4):399–415. doi: 10.1007/BF01024888. [DOI] [PubMed] [Google Scholar]
- Malke H. Codon usage in streptococci. J Basic Microbiol. 1986;26(10):587–595. doi: 10.1002/jobm.3620261006. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Park Y. H., Kim E., Yim D. G., Kho Y. H., Mheen T. I., Goodfellow M. Suprageneric classification of thermoactinomyces vulgaris by nucleotide sequencing of 5S ribosomal RNA. Zentralbl Bakteriol. 1993 Jun;278(4):469–478. doi: 10.1016/s0934-8840(11)80818-2. [DOI] [PubMed] [Google Scholar]
- Petrícek M., Tichý P., Kuncová M. Characterization of the alpha-amylase-encoding gene from Thermomonospora curvata. Gene. 1992 Mar 1;112(1):77–83. doi: 10.1016/0378-1119(92)90305-9. [DOI] [PubMed] [Google Scholar]
- Rogers J. C. Conserved amino acid sequence domains in alpha-amylases from plants, mammals, and bacteria. Biochem Biophys Res Commun. 1985 Apr 16;128(1):470–476. doi: 10.1016/0006-291x(85)91702-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonen M., Palva I. Protein secretion in Bacillus species. Microbiol Rev. 1993 Mar;57(1):109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E., Ludwig W., Weizenegger M., Dorn S., McGill T. J., Fox G. E., Woese C. R., Schubert W., Schleifer K. H. Comparative 16S rRNA oligonucleotide analyses and murein types of round-spore-forming bacilli and non-spore-forming relatives. J Gen Microbiol. 1987 Sep;133(9):2523–2529. doi: 10.1099/00221287-133-9-2523. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Kunst F., Dedonder R. Mapping of mutations affecting synthesis of exocellular enzymes in Bacillus subtilis. Identity of the sacUh, amyB and pap mutations. Mol Gen Genet. 1976 Nov 17;148(3):281–285. doi: 10.1007/BF00332902. [DOI] [PubMed] [Google Scholar]
- Svensson B. Regional distant sequence homology between amylases, alpha-glucosidases and transglucanosylases. FEBS Lett. 1988 Mar 28;230(1-2):72–76. doi: 10.1016/0014-5793(88)80644-6. [DOI] [PubMed] [Google Scholar]
- Takkinen K., Pettersson R. F., Kalkkinen N., Palva I., Söderlund H., Käriäinen L. Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. J Biol Chem. 1983 Jan 25;258(2):1007–1013. [PubMed] [Google Scholar]
- Tonozuka T., Ohtsuka M., Mogi S., Sakai H., Ohta T., Sakano Y. A neopullulanase-type alpha-amylase gene from Thermoactinomyces vulgaris R-47. Biosci Biotechnol Biochem. 1993 Mar;57(3):395–401. doi: 10.1271/bbb.57.395. [DOI] [PubMed] [Google Scholar]
- Uozumi N., Sakurai K., Sasaki T., Takekawa S., Yamagata H., Tsukagoshi N., Udaka S. A single gene directs synthesis of a precursor protein with beta- and alpha-amylase activities in Bacillus polymyxa. J Bacteriol. 1989 Jan;171(1):375–382. doi: 10.1128/jb.171.1.375-382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vihinen M., Mäntsälä P. Microbial amylolytic enzymes. Crit Rev Biochem Mol Biol. 1989;24(4):329–418. doi: 10.3109/10409238909082556. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsel S., Lachmund A., Wildhardt G., Ruttkowski E. Three alpha-amylase genes of Aspergillus oryzae exhibit identical intron-exon organization. Mol Microbiol. 1989 Jan;3(1):3–14. doi: 10.1111/j.1365-2958.1989.tb00097.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]