Abstract
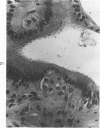
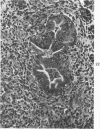
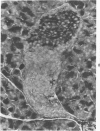
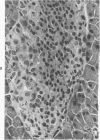
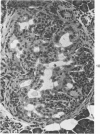
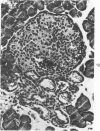
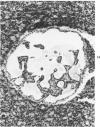
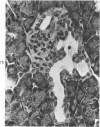
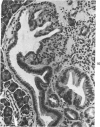
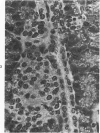
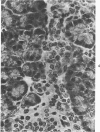
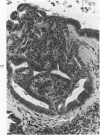
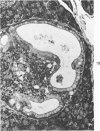
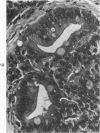
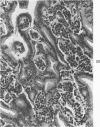
The ductular complex of the Syrian hamster pancreas represents a system of conduit which encompasses intercalated (intralobular), periinsular, and intrainsular ductules. The intercalated (intralobular) ductules comprise centroacinar and intercalated cells. A meshwork of small ductules (invisible by usual histologic procedures) surrounds islets (periinsular ductules) and extends in the form of often ramified tiny channels within the islet (intrainsular ductules). Although the function of the latter ductules is obscure, their cells seem to make up one of the undifferentiated cellular units of the pancreas, and as such are also the progenitors of beta-cells of the islets (islet cell precursor = IP). Systematic histologic examination of the pancreas in this species treated with pancreatic carcinogen N-nitrosobis(2-oxopropyl)amine indicated that ductular cells, especially those of periinsular and intrainsular origin, are the most responsive to this carcinogen. The neoplastic process was initiated with hyperplasia of intercalated (intralobular) ductular and interlobular ductal cells associated with newly formed islets (nesidioblastosis). This process was followed by excess formation of mature but especially of immature islet cells and their precursors (IP) in the islet periphery, as well as with the appearance, distention, and multiplication of periinsular and particularly of intrainsular ductules. The hyperplasia, metaplasia, and malignant alteration of these periinsular and intrainsular ductules (including IP) and, to a lesser degree, of intercalated ductules indicated their histogenetic relationship and their potency for reproducing embryonic tissue on carcinogenic stimulus. The similarity of some induced lesions to diabetes has been emphasized.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENCOSME S. A. The histogenesis and cytology of the pancreatic islets in the rabbit. Am J Anat. 1955 Jan;96(1):103–151. doi: 10.1002/aja.1000960105. [DOI] [PubMed] [Google Scholar]
- CRANE W. A., DUTTA L. P. THE INFLUENCE OF AGE AND HORMONAL STATUS ON THE UPTAKE OF TRITIATED THYMIDINE BY RAT PANCREAS. J Endocrinol. 1964 Feb;28:341–342. doi: 10.1677/joe.0.0280341. [DOI] [PubMed] [Google Scholar]
- D'Aunoy R., Ogden M. A., Halpert B. Carcinoma of the pancreas: An analysis of forty autopsies. Am J Pathol. 1939 Mar;15(2):217–224.1. [PMC free article] [PubMed] [Google Scholar]
- FORSHALL I., RICKHAM P. P., HALL E. G. An unusual islet-cell tumour of the pancreas. Br J Surg. 1952 Sep;40(160):181–184. doi: 10.1002/bjs.18004016023. [DOI] [PubMed] [Google Scholar]
- GLENNER G. G., MALLORY G. K. The cystadenoma and related nonfunctional tumors of the pancreas; pathogenesis, classification, and significance. Cancer. 1956 Sep-Oct;9(5):980–996. doi: 10.1002/1097-0142(195609/10)9:5<980::aid-cncr2820090519>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- GOMORI G. Aldehyde-fuchsin: a new stain for elastic tissue. Am J Clin Pathol. 1950 Jul;20(7):665–666. [PubMed] [Google Scholar]
- Gomori G. Observations with differential stains on human islets of langerhans. Am J Pathol. 1941 May;17(3):395–406.3. [PMC free article] [PubMed] [Google Scholar]
- HOUSE E. L., NACE P. F., TASSONI J. P. Alloxan diabetes in the hamster: organ changes during the first day. Endocrinology. 1956 Oct;59(4):433–443. doi: 10.1210/endo-59-4-433. [DOI] [PubMed] [Google Scholar]
- Hajdu A., Herr F., Rona G. The functional significance of a spontaneous pancreatic islet change in aged rats. Diabetologia. 1968 Jan;4(1):44–47. doi: 10.1007/BF01241032. [DOI] [PubMed] [Google Scholar]
- Hughes H. Cyclical changes in the islets of Langerhans in the rat pancreas. J Anat. 1947 Jan;81(Pt 1):82–92.2. [PMC free article] [PubMed] [Google Scholar]
- Laidlaw G. F. Nesidioblastoma, the islet tumor of the pancreas. Am J Pathol. 1938 Mar;14(2):125–134.5. [PMC free article] [PubMed] [Google Scholar]
- Mainz D., Webster P. D., 3rd Pancreatic carcinoma. A review of etiologic considerations. Am J Dig Dis. 1974 May;19(5):459–464. doi: 10.1007/BF01255609. [DOI] [PubMed] [Google Scholar]
- Melmed R. N., Benitez C. J., Holt S. J. Intermediate cells of the pancreas. I. Ultrastructural characterization. J Cell Sci. 1972 Sep;11(2):449–475. doi: 10.1242/jcs.11.2.449. [DOI] [PubMed] [Google Scholar]
- NERENBERG S. T. Beta granules of islets of Langerhans of rat; a study of their development under normal and abnormal conditions. AMA Arch Pathol. 1954 Sep;58(3):236–240. [PubMed] [Google Scholar]
- POEL W. E., YERGANIAN G. Adenocarcinoma of the pancreas in diabetes-prone Chinese hamsters. Am J Med. 1961 Dec;31:861–863. doi: 10.1016/0002-9343(61)90026-2. [DOI] [PubMed] [Google Scholar]
- Patent G. J., Alfert M. Histological changes in the pancreatic islets of alloxan-treated mice, with comments on beta-cell regeneration. Acta Anat (Basel) 1967;66(4):504–519. doi: 10.1159/000142962. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Pour P., Althoff J., Krüger F. W., Mohr U. A potent pancreatic carcinogen in Syrian hamsters: N-nitrosobis(2-oxopropyl)amine. J Natl Cancer Inst. 1977 May;58(5):1449–1453. doi: 10.1093/jnci/58.5.1449. [DOI] [PubMed] [Google Scholar]
- Pour P., Althoff J., Takahashi M. Early lesions of pancreatic ductal carcinoma in the hamster model. Am J Pathol. 1977 Aug;88(2):291–308. [PMC free article] [PubMed] [Google Scholar]
- Pour P., Althoff J., Takahashi M. Early lesions of pancreatic ductal carcinoma in the hamster model. Am J Pathol. 1977 Aug;88(2):291–308. [PMC free article] [PubMed] [Google Scholar]
- Pour P., Krüger F. W., Althoff J., Cardesa A., Mohr U. Cancer of the pancreas induced in the Syrian golden hamster. Am J Pathol. 1974 Aug;76(2):349–358. [PMC free article] [PubMed] [Google Scholar]
- Pour P., Mohr U., Cardesa A., Althoff J., Krüger F. W. Pancreatic neoplasms in an animal model: morphological, biological, and comparative studies. Cancer. 1975 Aug;36(2):379–389. doi: 10.1002/1097-0142(197508)36:2<379::aid-cncr2820360213>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- SOMMERS S. C., MEISSNER W. A. Unusual carcinomas of the pancreas. AMA Arch Pathol. 1954 Aug;58(2):101–111. [PubMed] [Google Scholar]
- Shino A., Iwatsuka H. Morphological observations on pancreatic islets of spontaneous diabetic mice, "Yellow KK". Endocrinol Jpn. 1970 Dec;17(6):459–476. doi: 10.1507/endocrj1954.17.459. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Pour P., Althoff J., Donnelly T. The pancreas of the Syrian hamster (Mesocricetus auratus). I Anatomical study. Lab Anim Sci. 1977 Jun;27(3):336–342. [PubMed] [Google Scholar]