Abstract
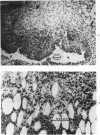
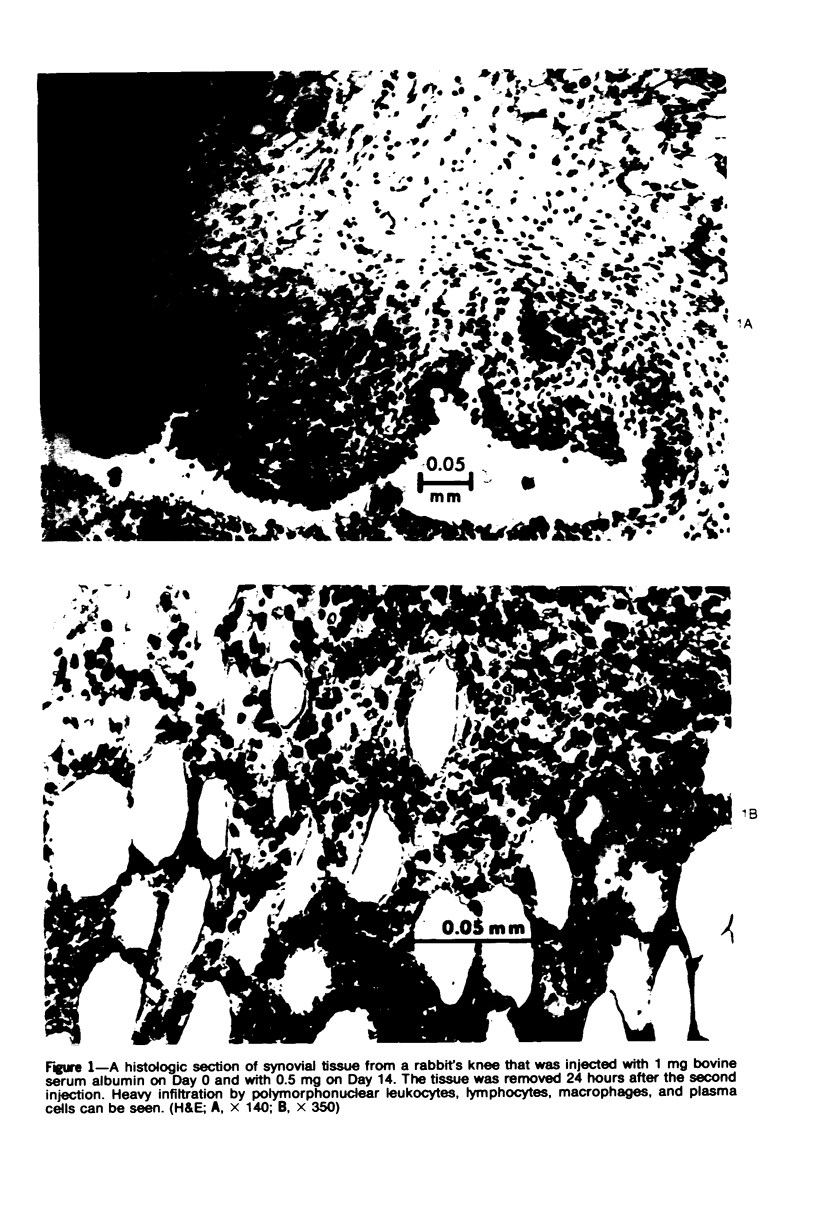
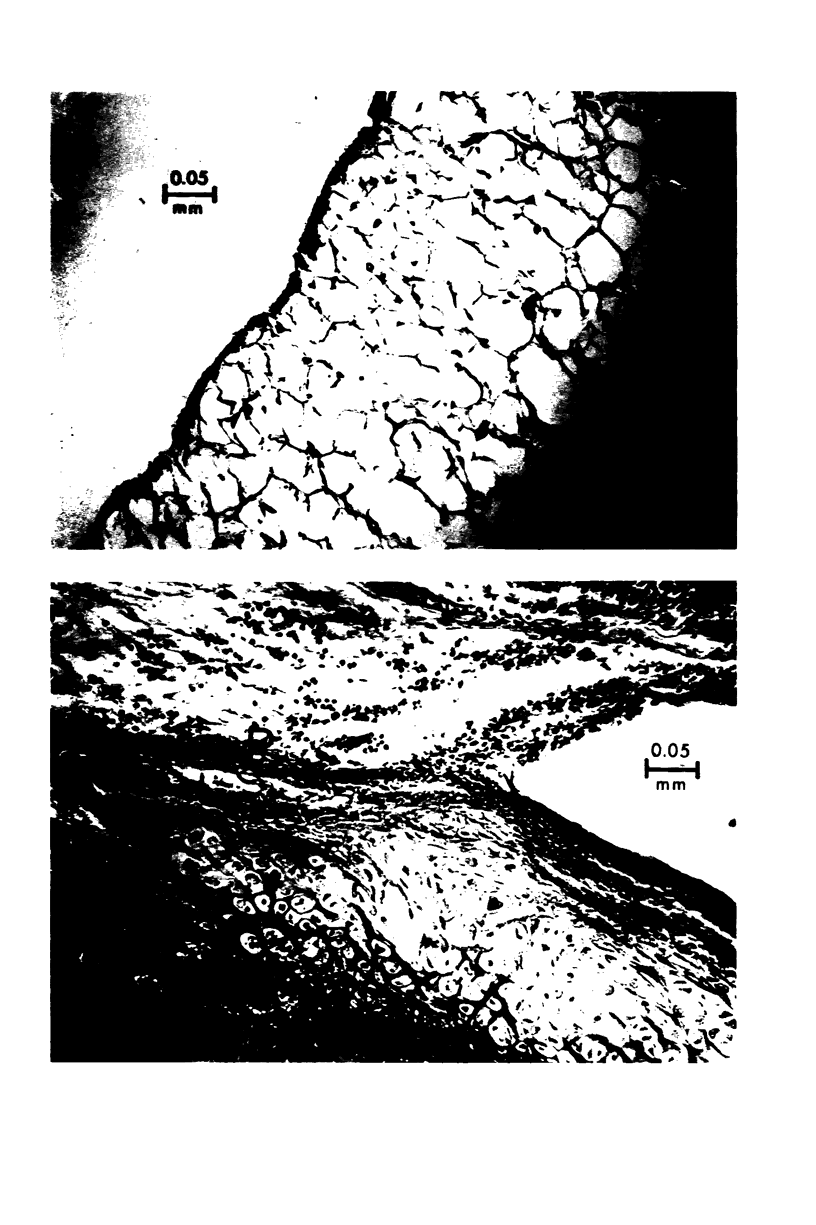
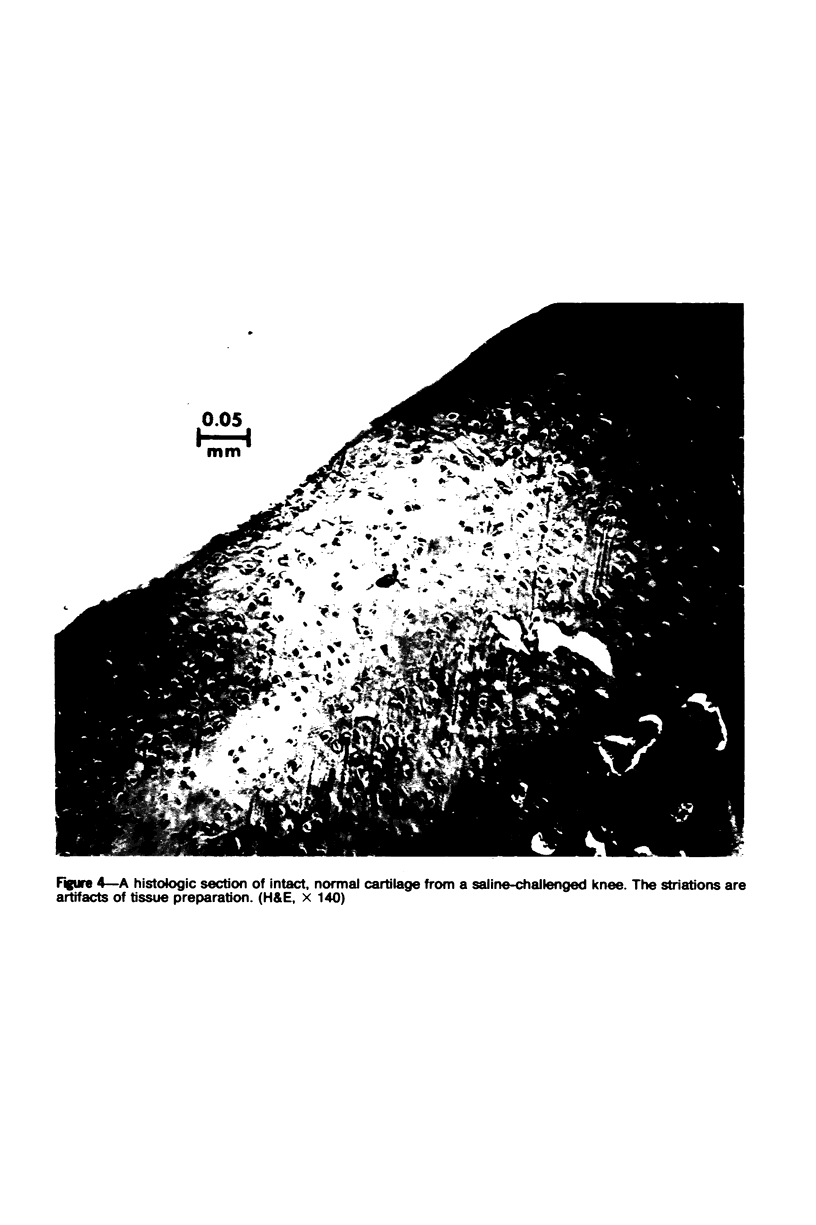
The effects of varying intra-articular (ia) doses of bovine serum albumin (BSA) antigen on immune synovitis in rabbits have been investigated. Chronic synovitis, characterized by mononuclear cell infiltration in synovial tissues, was induced by a single ia challenge with BSA in sensitized rabbits. However, cartilage and bone erosions and pannus formation were rarely observed. By varying the number and magnitude of the BSA challenges, lesions with different characteristics were observed at different times of analysis of joint pathology. In 3- to 10-week studies, multiple ia challenges with BSA produced lesions characterized by severe cartilage and bone changes; polymorphonuclear leukocyte (PMN) exudates; and mononuclear cells and, sometimes, PMNs in synovial tissues. Substantial increases in knee widths and synovial tissue weights also observed. By increasing the frequency of ia injections, more severe changes were produced more rapidly, so that within a 3-week period, the animals also experienced pain and were unable to fully extend their antigen-challenged knees. Some of the lesions observed in immune synovis resembled those in rheumatoid arthritis (RA). However, the presence of large numbers of PMNs in synovial tissue under certain conditions suggests some possible differences between the pathogenesis of experimental synovitis and RA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke T. D., Hurd E. R., Jasin H. E., Bienenstock J., Ziff M. Identification of immunoglobulins and complement in rheumatoid articular collagenous tissues. Arthritis Rheum. 1975 Nov-Dec;18(6):541–551. doi: 10.1002/art.1780180603. [DOI] [PubMed] [Google Scholar]
- Cooke T. D., Hurd E. R., Ziff M., Jasin H. E. The pathogenesis of chronic inflammation in experimental antigen-induced arthritis. II. Preferential localization of antigen-antibody complexes to collagenous tissues. J Exp Med. 1972 Feb 1;135(2):323–338. doi: 10.1084/jem.135.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Fox A., Glynn L. E. Is persisting antigen responsible for the chronicity of experimental allergic arthritis? Ann Rheum Dis. 1977 Feb;36(1):34–38. doi: 10.1136/ard.36.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg V. M., Lance E. M., Davis P. Experimental immune synovitis in the rabbit. Relative roles of cell mediated and humoral immunity. Arthritis Rheum. 1974 Nov-Dec;17(6):993–1005. doi: 10.1002/art.1780170612. [DOI] [PubMed] [Google Scholar]
- Goldlust M. B., Rich L. C., Harrity T. W. Effects of antiinflammatory agents on the acute response of immune synovitis in rabbits. Arthritis Rheum. 1977 May;20(4):937–946. doi: 10.1002/art.1780200406. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Shannon S. L. Peroxidase arthritis. 3. Sequential changes in cellular composition of the developing inflammatory infiltrate. Am J Pathol. 1973 Oct;73(1):147–156. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Shannon S. L. Peroxidase arthritis. I. An immunologically mediated inflammatory response with ultrastructural cytochemical localization of antigen and specific antibody. Am J Pathol. 1972 Apr;67(1):69–94. [PMC free article] [PubMed] [Google Scholar]
- Jasin H. E., Ziff M. Immunoglobulin and specific antibody synthesis in a chronic inflammatory focus: antigen-induced synovitis. J Immunol. 1969 Feb;102(2):355–369. [PubMed] [Google Scholar]
- Ménard H. A., Demers J. C. Use of a hapten-carrier system in experimental immune arthritis in the rabbit. Arthritis Rheum. 1977 Sep-Oct;20(7):1402–1408. doi: 10.1002/art.1780200715. [DOI] [PubMed] [Google Scholar]
- Müller P., Raabe G., Thoss K. BSA-induced allergic arthritis and formol-induced non-allergic arthritis in rabbits. 1. Comparative studies on morphological features and course of the disease. Exp Pathol (Jena) 1973;8(5):331–340. [PubMed] [Google Scholar]
- Stastny P., Cooke T. D., Ziff M. Production of a macrophage migration inhibitory factor in rabbits with experimental arthritis. Clin Exp Immunol. 1973 May;14(1):141–147. [PMC free article] [PubMed] [Google Scholar]
- Stastny P., Rosenthal M., Andreis M., Ziff M. Lymphokines in the rheumatoid joint. Arthritis Rheum. 1975 May-Jun;18(3):237–243. doi: 10.1002/art.1780180307. [DOI] [PubMed] [Google Scholar]
- Steinberg M. E., McCrae C. R., Cohen L. D., Schumacher H. R., Jr Pathogenesis of antigen-induced arthritis. Clin Orthop Relat Res. 1973 Nov-Dec;(97):248–260. doi: 10.1097/00003086-197311000-00031. [DOI] [PubMed] [Google Scholar]