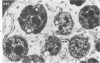
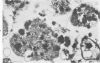
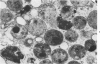
Abstract
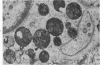
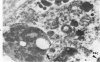
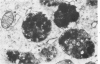
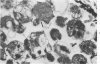
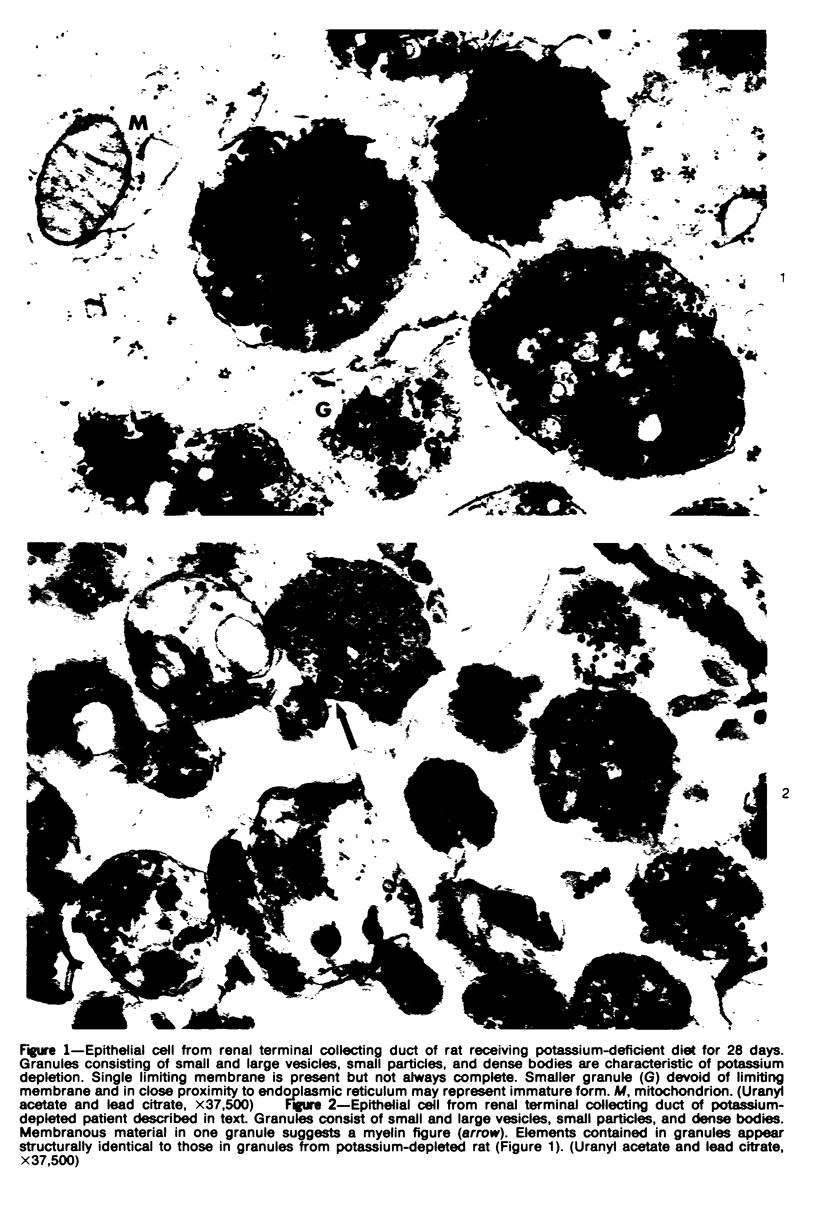
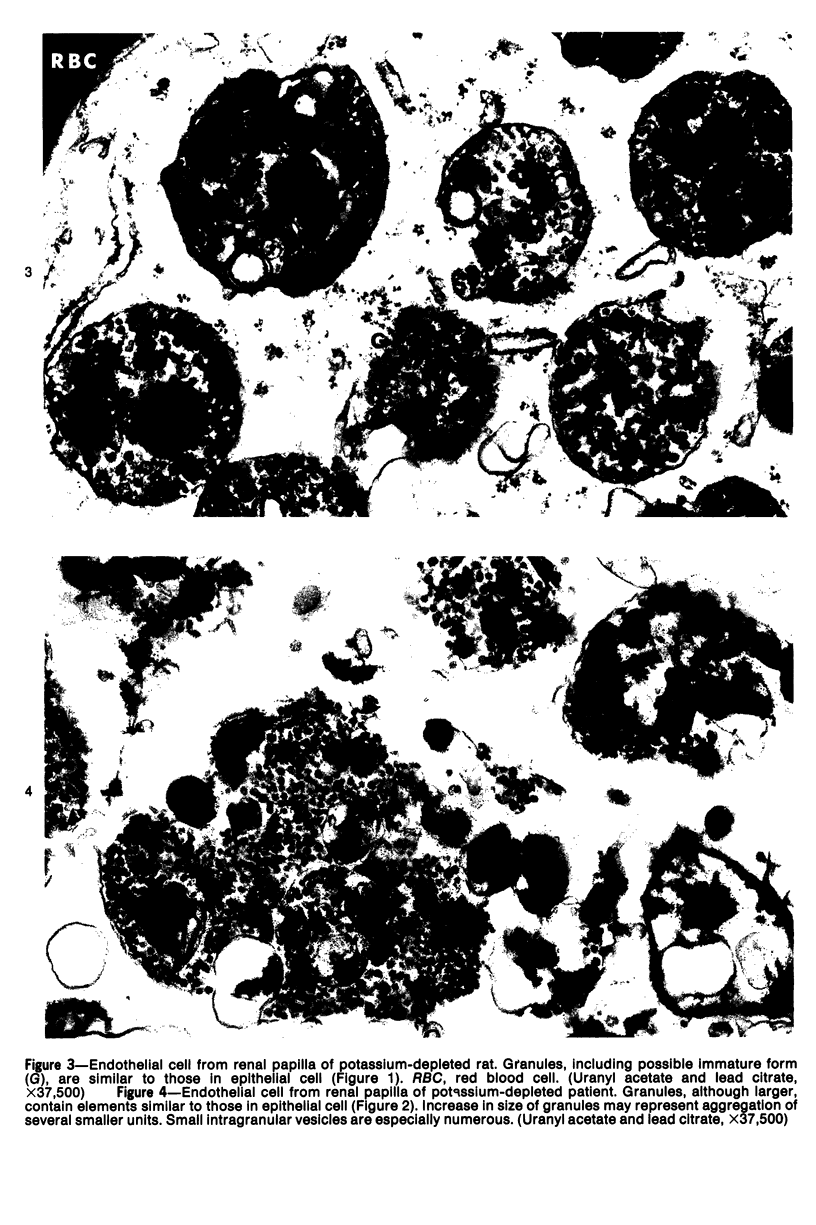
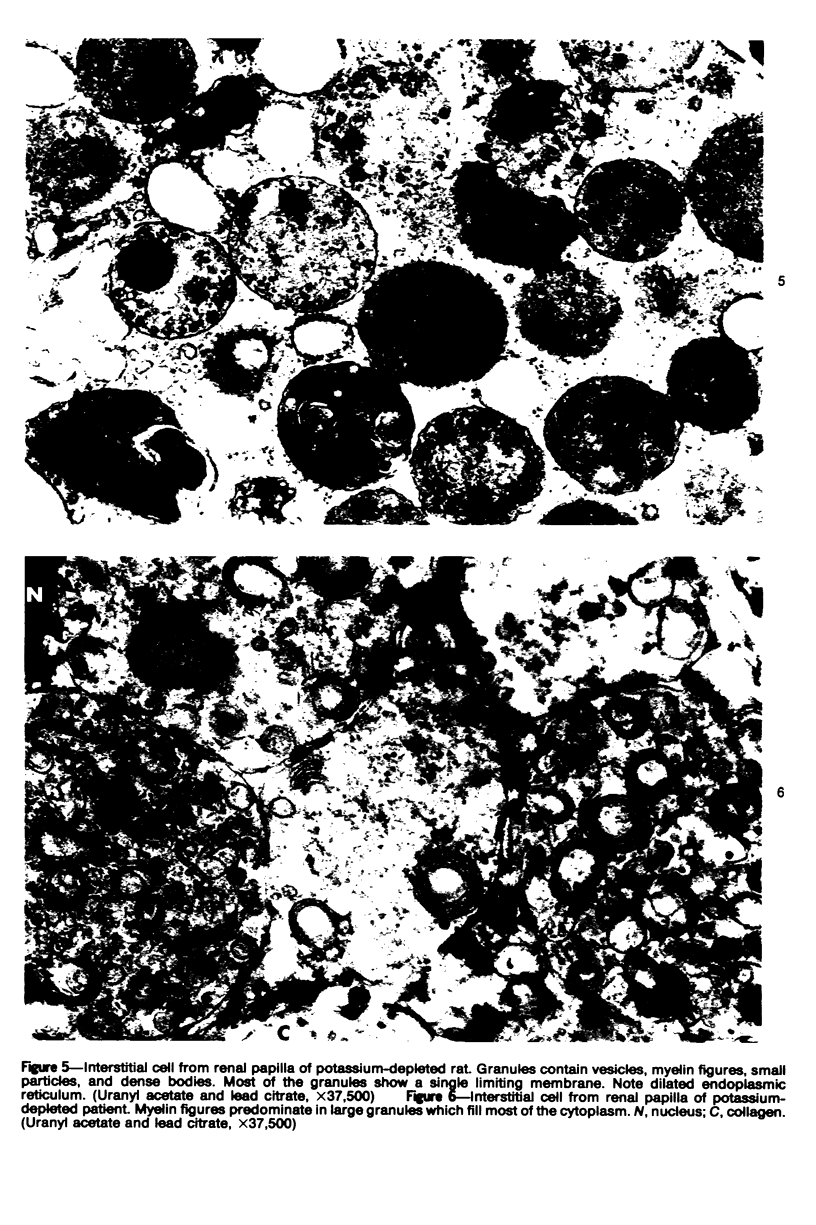
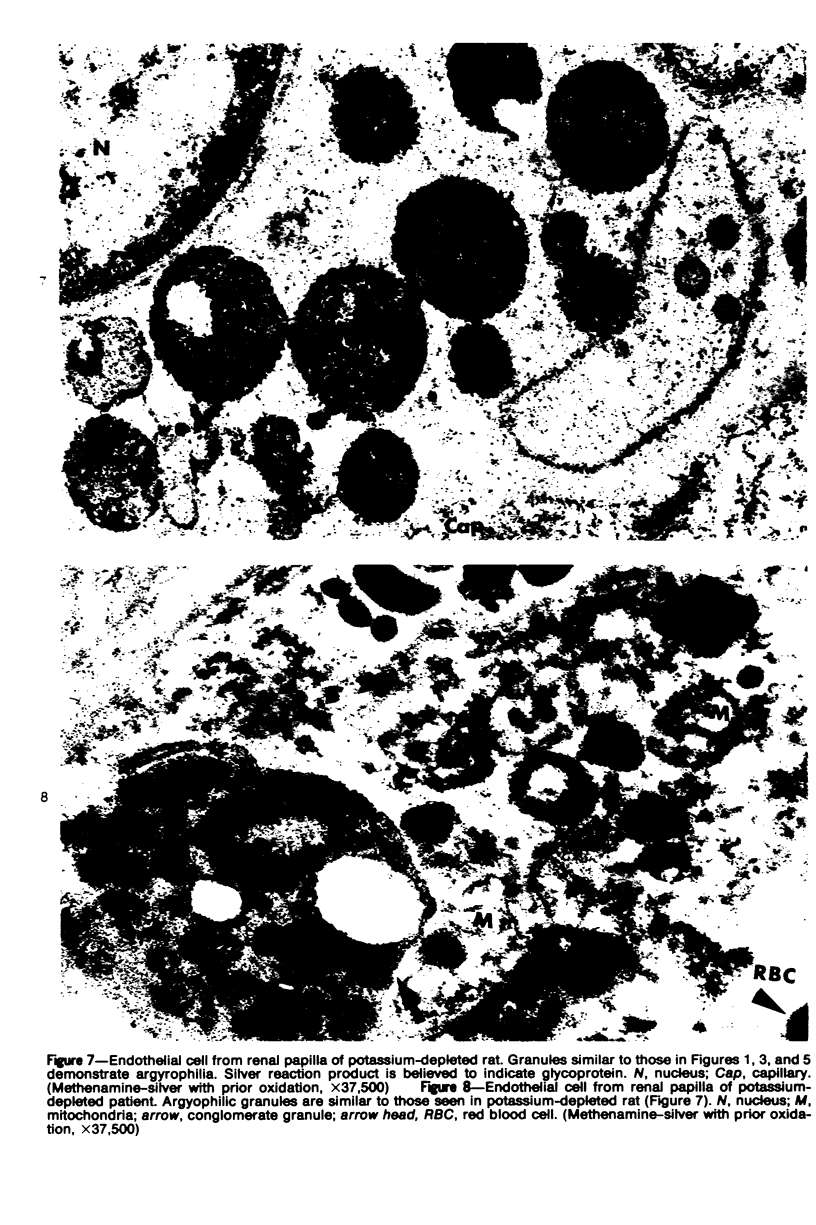
An electron microscopic comparison was made of intracellular granules of the renal papilla and inner medulla in two types of potassium depletion: one in a 47-year-old white male with chronic potassium-wasting renal disease and the other in the experimentally depleted rat. The granules in both cases were composed of small and large vesicles; myelin figures; small particles; and dense bodies, with a partial, or complete, single limiting membrane. Ultrastructurally, the constituent elements of the granules were essentially the same in the two types of potassium depletion. It was concluded that the intracellular granules in the human tissue were the result of potassium depletion and a counterpart to those in the potassium-depleted rat.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aithal H. N., Toback F. G., Dube S., Getz G. S., Spargo B. H. Formation of renal medullary lysosomes during potassium depletion nephropathy. Lab Invest. 1977 Feb;36(2):107–113. [PubMed] [Google Scholar]
- BARTTER F. C., PRONOVE P., GILL J. R., Jr, MACCARDLE R. C. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med. 1962 Dec;33:811–828. doi: 10.1016/0002-9343(62)90214-0. [DOI] [PubMed] [Google Scholar]
- Bartter F. C., Delea C. S., Kawasaki T., Gill J. R., Jr The adrenal cortex and the kidney. Kidney Int. 1974 Nov;6(5):272–280. doi: 10.1038/ki.1974.113. [DOI] [PubMed] [Google Scholar]
- Bartter F. C., Gill J. R., Jr, Frolich J. C., Bowden R. E., Hollifield J. W., Radfar N., Keiser H. R., Oates J. A., Seyberth H., Taylor A. A. Prostaglandins are overproduced by the kidneys and mediate hyperreninemia in Bartter's syndrome. Trans Assoc Am Physicians. 1976;89:77–91. [PubMed] [Google Scholar]
- CRAIG J. M., SCHWARTZ R. Histochemical study of the kidney of rats fed diets deficient in potassium. AMA Arch Pathol. 1957 Sep;64(3):245–254. [PubMed] [Google Scholar]
- Erkelens D. W., Statius van Eps L. W. Bartter's syndrome and erythrocytosis. Am J Med. 1973 Nov;55(5):711–719. doi: 10.1016/0002-9343(73)90196-4. [DOI] [PubMed] [Google Scholar]
- FRANCE R. INTRACELLULAR GRANULES OF THE RENAL MEDULLA IN POTASSIUM DEPLETION. Trans Am Clin Climatol Assoc. 1962;74:211–230. [PMC free article] [PubMed] [Google Scholar]
- Fichman M. P., Telfer N., Zia P., Speckart P., Golub M., Rude R. Role of prostaglandins in the pathogenesis of Bartter's syndrome. Am J Med. 1976 May 31;60(6):785–797. doi: 10.1016/0002-9343(76)90892-5. [DOI] [PubMed] [Google Scholar]
- France R., Shelley W. M., Gray M. E. Abnormal intracellular granules of the renal papilla in a child with potassium depletion (Bartter's syndrome) and renal tuberous sclerosis. Johns Hopkins Med J. 1974 Oct;135(4):274–285. [PubMed] [Google Scholar]
- France R., Stone W. J., Michelakis A. M., Island D. P., Merrill J. M., Tolleson W. J. Renal potassium-wasting of unknown cause in a clinical setting of chronic potassium depletion: report of a case. South Med J. 1973 Jan;66(1):115–128. doi: 10.1097/00007611-197301000-00020. [DOI] [PubMed] [Google Scholar]
- HOLLANDER W., Jr, WINTERS R. W., WILLIAMS T. F., BRADLEY J., OLIVER J., WELT L. G. Defect in the renal tubular reabsorption of water associated with potassium depletion in rats. Am J Physiol. 1957 Jun;189(3):557–563. doi: 10.1152/ajplegacy.1957.189.3.557. [DOI] [PubMed] [Google Scholar]
- Hruban Z., Slesers A., Hopkins E. Drug-induced and naturally occurring myeloid bodies. Lab Invest. 1972 Jul;27(1):62–70. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON A. B., PANNER B. J. LYSOSOME INDUCTION IN EXPERIMENTAL POTASSIUM DEFICIENCY. Am J Pathol. 1964 Aug;45:295–311. [PMC free article] [PubMed] [Google Scholar]
- Ordóez N. G., Toback F. G., Aithal H. N., Spargo B. J. Zonal changes in renal structure and phospholipid metabolism during reversal of potassium depletion nephropathy. Lab Invest. 1977 Jan;36(1):33–47. [PubMed] [Google Scholar]
- Panner B. J. Ultrastructural and histochemical observations on developing lysosomes in experimental hypokalemia. Studies on tissue sections and on homogenates. Lab Invest. 1971 Jul;25(1):53–59. [PubMed] [Google Scholar]
- RELMAN A. S., SCHWARTZ W. B. The nephropathy of potassium depletion; a clinical and pathological entity. N Engl J Med. 1956 Aug 2;255(5):195–203. doi: 10.1056/NEJM195608022550501. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A., Hernandez W., Leblond C. P. Detection of complex carbohydrates in the Golgi apparatus of rat cells. J Cell Biol. 1969 Feb;40(2):395–414. doi: 10.1083/jcb.40.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPARGO B. Kidney changes in hypokalemic alkalosis in the rat. J Lab Clin Med. 1954 May;43(5):802–814. [PubMed] [Google Scholar]
- Simopoulos A. P., Bartter F. C. Growth characteristics and factors influencing growth in Bartter's syndrome. J Pediatr. 1972 Jul;81(1):56–65. doi: 10.1016/s0022-3476(72)80374-3. [DOI] [PubMed] [Google Scholar]
- Toback F. G., Ordónez N. G., Bortz S. L., Spargo B. H. Zonal changes in renal structure and phospholipid metabolism in potassium-deficient rats. Lab Invest. 1976 Feb;34(2):115–124. [PubMed] [Google Scholar]
- Verberckmoes R., van Damme B B., Clement J., Amery A., Michielsen P. Bartter's syndrome with hyperplasia of renomedullary cells: successful treatment with indomethacin. Kidney Int. 1976 Mar;9(3):302–307. doi: 10.1038/ki.1976.33. [DOI] [PubMed] [Google Scholar]
- Wilson H., Spargo B., Getz G. S. Changes in kidney medullary phospholipid metabolism in the potassium-deficient rat. I. Am J Pathol. 1973 May;71(2):295–314. [PMC free article] [PubMed] [Google Scholar]
- Wilson H., Spargo B., Getz G. S. Changes in kidney medullary phospholipid metabolism in the potassium-depleted rat. II. Am J Pathol. 1973 May;71(2):315–330. [PMC free article] [PubMed] [Google Scholar]