Abstract
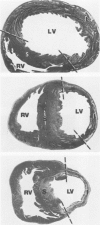
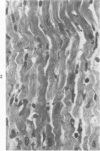
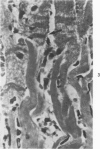
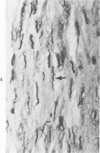
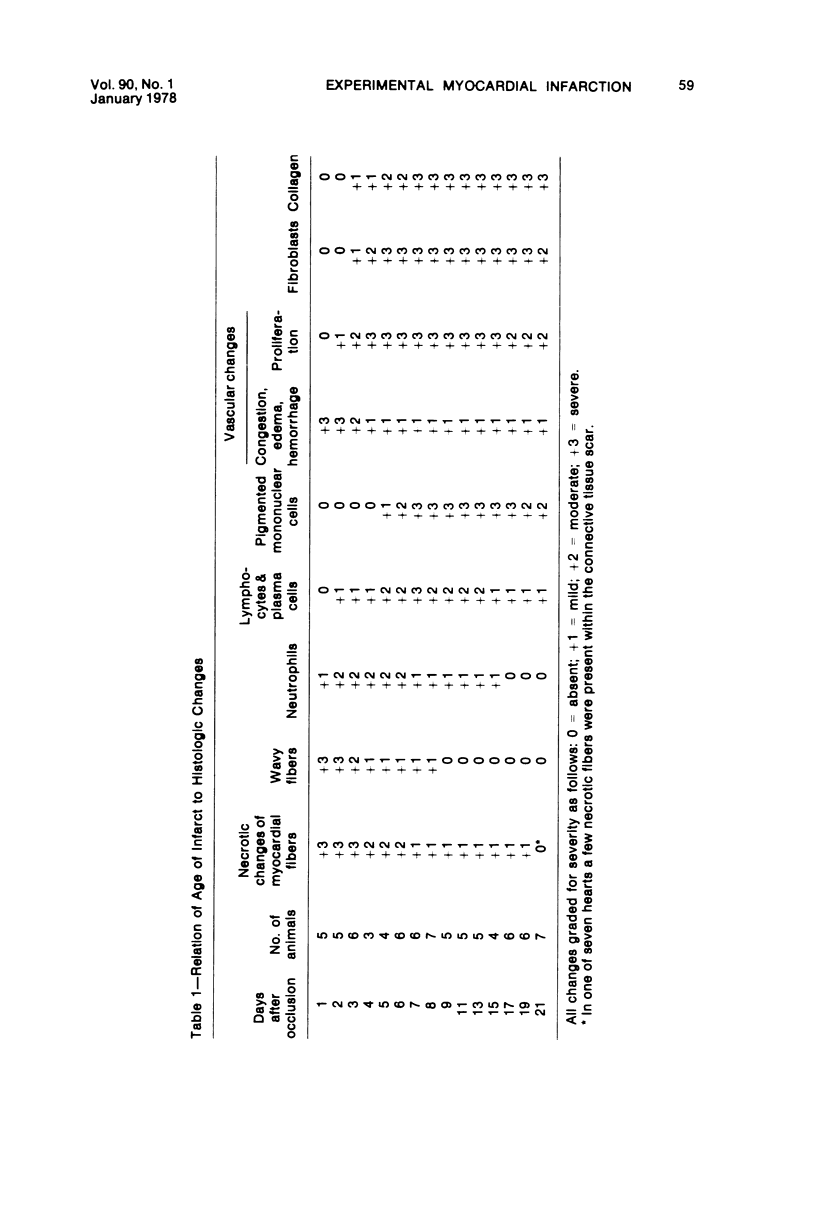
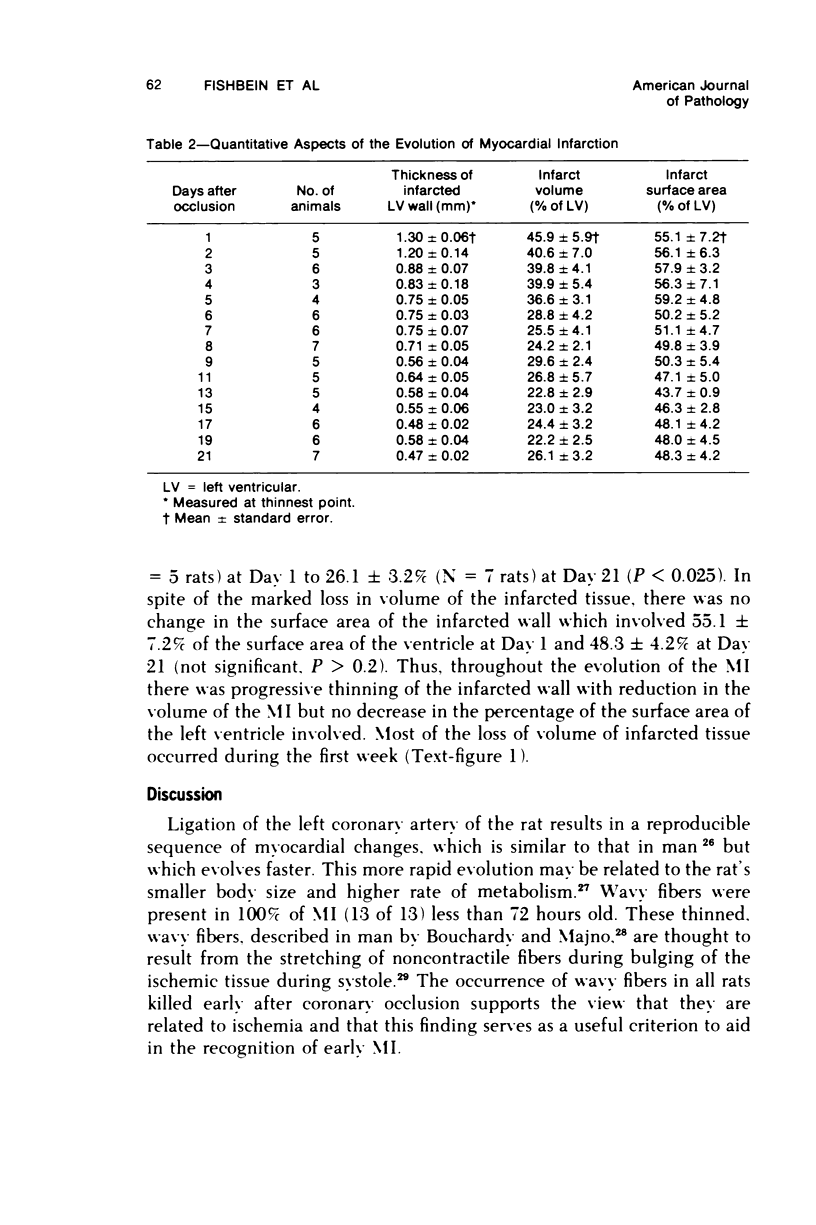
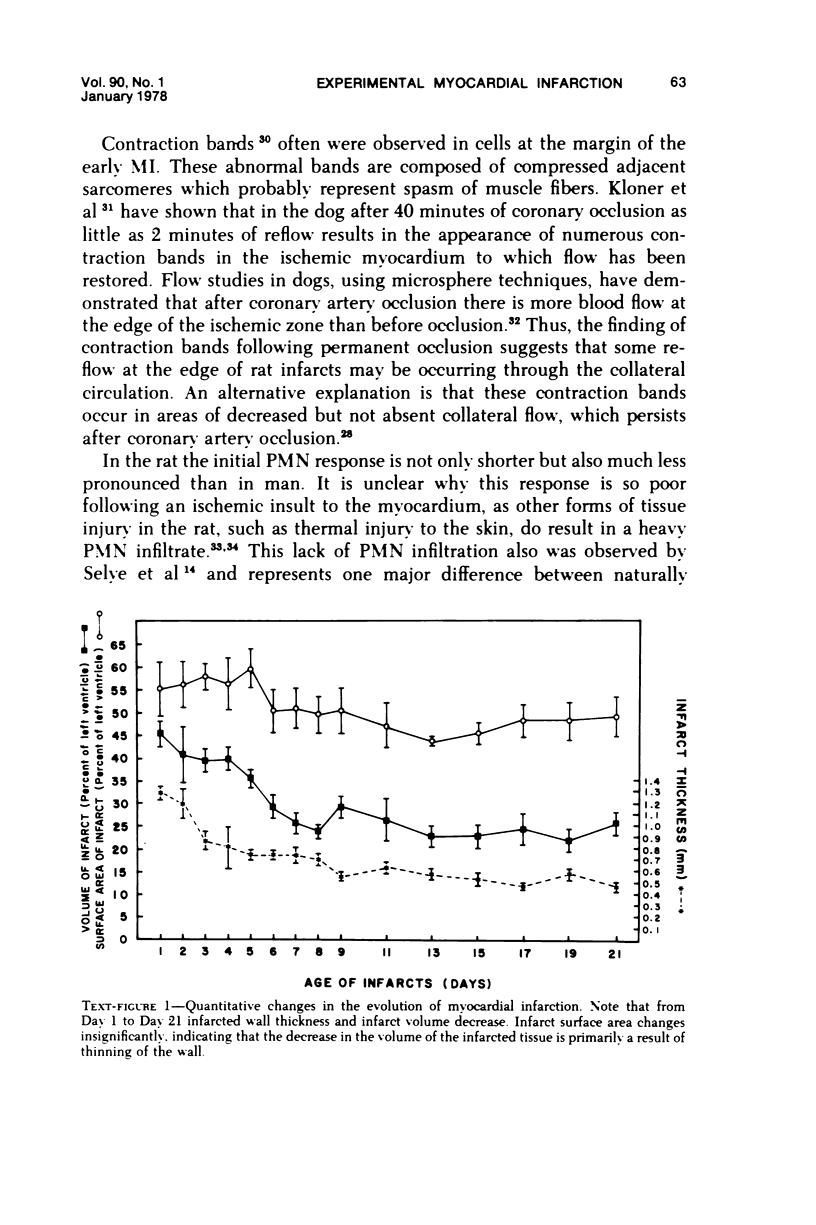
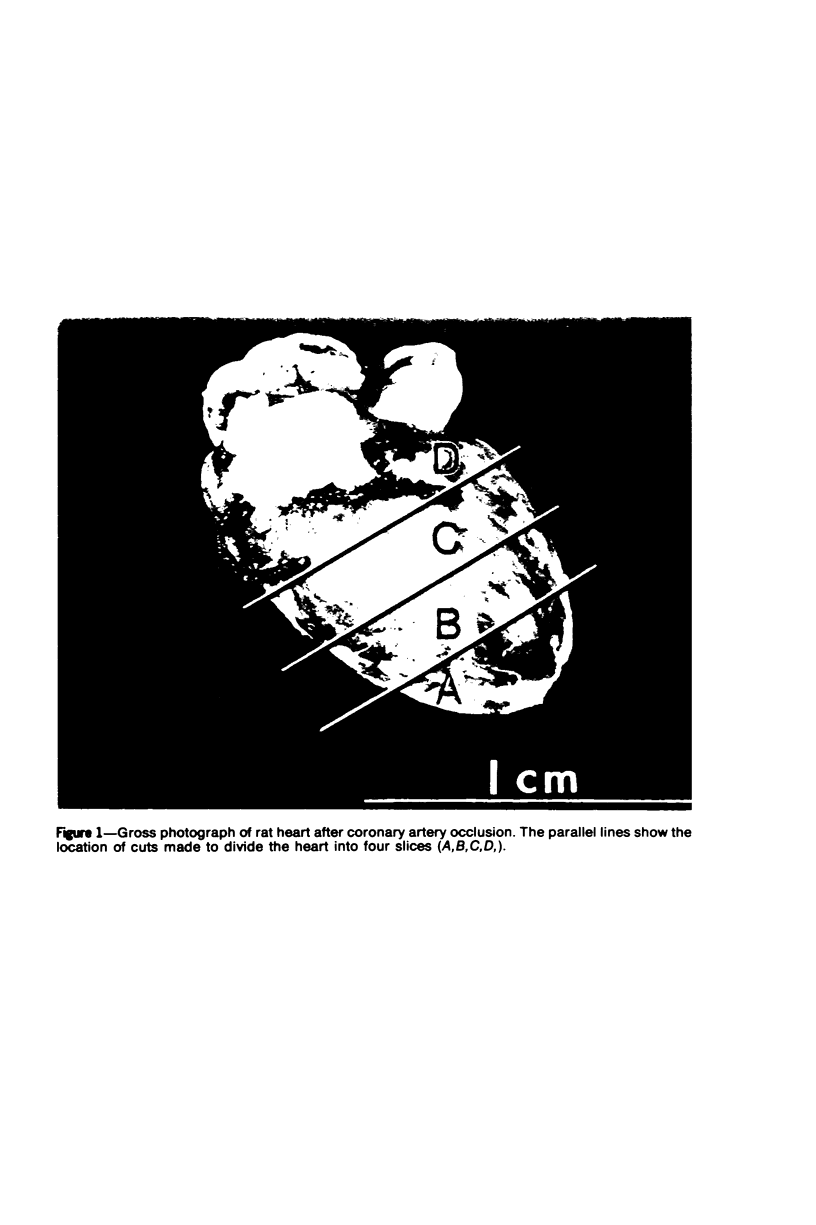
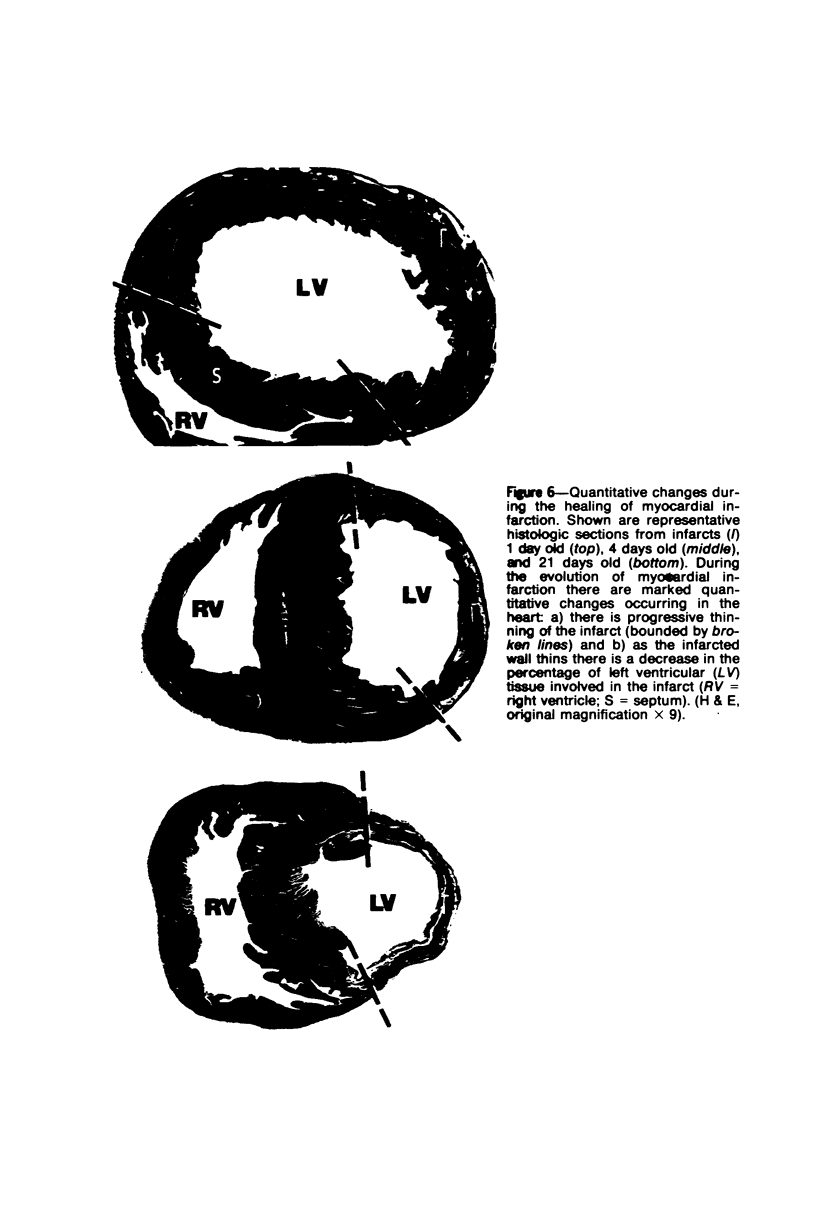
Surgical occlusion of the left coronary artery of the rat is a relatively simple, economical technique for producing experimental myocardial infarction (MI). Histologic study of 1- to 21-day-old MI in rats showed that following a mild and brief acute inflammatory response at the margins of the necrotic myocardium, there is chronic inflammation, vascular and collagenous proliferation, and resorption of necrostic tissue which progresses until scar formation is complete, usually by 21 days. From Day 1 to Day 21 the volume of infarcted myocardium decreases from 45.9 +/- 5.9% (mean +/- SEM) to 26.1 +/- 3.2% of the left ventricle and infarct thickness decreases from 1.30 +/- 0.06 mm to 0.47 +/- 0.02 mm. Concomitantly, the percent of the surface area of the left ventricle which is infarcted decreases insignificantly from 55.7 +/- 7.2% to 48.3 +/- 4.2%, indicating that the decrease in volume of the infarcted tissue occurs primarily as a result of thinning of the MI. This study provides qualitative and quantitative information on the natural history of MI in rats, which should be useful as a baseline for future studies.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAJUSZ E., JASMIN G. HISTOCHEMICAL STUDIES ON THE MYOCARDIUM FOLLOWING EXPERIMENTAL INTERFERENCE WITH CORONARY CIRCULATION IN THE RAT. I. OCCLUSION OF CORONARY ARTERY. Acta Histochem. 1964 Jun 30;18:222–237. [PubMed] [Google Scholar]
- Baroldi G. Different types of myocardial necrosis in coronary heart disease: a pathophysiologic review of their functional significance. Am Heart J. 1975 Jun;89(6):742–752. doi: 10.1016/0002-8703(75)90189-1. [DOI] [PubMed] [Google Scholar]
- Bishop S. P., White F. C., Bloor C. M. Regional myocardial blood flow during acute myocardial infarction in the conscious dog. Circ Res. 1976 May;38(5):429–438. doi: 10.1161/01.res.38.5.429. [DOI] [PubMed] [Google Scholar]
- Camilleri J. P., Fabiani J. N., Deloche A., Gurdjian C. Etude histochimique et histoenzymologique de l'enfarctus expérimental du rat apreès ligature permanente ou temporaire de la coronaire gauche. Virchows Arch A Pathol Anat Histol. 1975;366(2):149–175. doi: 10.1007/BF00433588. [DOI] [PubMed] [Google Scholar]
- Deloche A., Fontaliran F., Fabiani J. N., Pennecot G., Carpentier A., Dubost C. Etude expérimentale de la revascularisation chirurgicale précoce de l'infarctus du myocarde. Ann Chir Thorac Cardiovasc. 1972 Jan;11(1):89–105. [PubMed] [Google Scholar]
- Dusek J., Rona G., Kahn D. S. Healing process in the marginal zone of an experimental myocardial infarct. Findings in the surviving cardiac muscle cells. Am J Pathol. 1971 Mar;62(3):321–338. [PMC free article] [PubMed] [Google Scholar]
- Epstein S. E., Kent K. M., Goldstein R. E., Borer J. S., Redwood D. R. Reduction of ischemic injury by nitroglycerin during acute myocardial infarction. N Engl J Med. 1975 Jan 2;292(1):29–35. doi: 10.1056/NEJM197501022920107. [DOI] [PubMed] [Google Scholar]
- HURLEY J. V., SPECTOR W. G. Delayed leucocytic emigration after intra-dermal injections and thermal injury. J Pathol Bacteriol. 1961 Oct;82:421–429. doi: 10.1002/path.1700820219. [DOI] [PubMed] [Google Scholar]
- HURLEY J. V., SPECTOR W. G. Endogenous factors responsible for leucocytic emigration in vivo. J Pathol Bacteriol. 1961 Oct;82:403–420. doi: 10.1002/path.1700820218. [DOI] [PubMed] [Google Scholar]
- JOHNS T. N. P., OLSON B. J. Experimental myocardial infarction. I. A method of coronary occlusion in small animals. Ann Surg. 1954 Nov;140(5):675–682. doi: 10.1097/00000658-195411000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN N., GAVAN T. L., HILL R. W. Experimental myocardial infarction in the rat. AMA Arch Pathol. 1959 May;67(5):482–488. [PubMed] [Google Scholar]
- Kloner R. A., Fishbein M. C., Cotran R. S., Braunwald E., Maroko P. R. The effect of propranolol on microvascular injury in acute myocardial ischemia. Circulation. 1977 Jun;55(6):872–880. doi: 10.1161/01.cir.55.6.872. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Fishbein M. C., Maclean D., Braunwald E., Maroko P. R. Effect of hyaluronidase during the early phase of acute myocardial ischemia: an ultrastructural and morphometric analysis. Am J Cardiol. 1977 Jul;40(1):43–49. doi: 10.1016/0002-9149(77)90098-4. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Maroko P. R., Bloor C. M., Sobel B. E., Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J Clin Invest. 1973 Mar;52(3):599–607. doi: 10.1172/JCI107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean D., Fishbein M. C., Maroko P. R., Braunwald E. Hyaluronidase-induced reductions in myocardial infarct size. Science. 1976 Oct 8;194(4261):199–200. doi: 10.1126/science.959848. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Hillis L. D., Muller J. E., Tavazzi L., Heyndrickx G. R., Ray M., Chiariello M., Distante A., Askenazi J., Salerno J. Favorable effects of hyaluronidase on electrocardiographic evidence of necrosis in patients with acute myocardial infarction. N Engl J Med. 1977 Apr 21;296(16):898–903. doi: 10.1056/NEJM197704212961603. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Kjekshus J. K., Sobel B. E., Watanabe T., Covell J. W., Ross J., Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971 Jan;43(1):67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Bloor C. M., Sobel B. E., Braunwald E. Reduction by hyaluronidase of myocardial necrosis following coronary artery occlusion. Circulation. 1972 Sep;46(3):430–437. doi: 10.1161/01.cir.46.3.430. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Braunwald E. Effect of pharmacologic agents on the function of the ischemic heart. Am J Cardiol. 1973 Dec;32(7):930–936. doi: 10.1016/s0002-9149(73)80160-2. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Ginks W. R., Bloor C. M., Shell W. E., Sobel B. E., Ross J., Jr Coronary artery reperfusion. I. Early effects on local myocardial function and the extent of myocardial necrosis. J Clin Invest. 1972 Oct;51(10):2710–2716. doi: 10.1172/JCI107090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachlas M. M., Siedband M. P. The influence of diastolic augmentation on infarct size following coronary artery ligation. J Thorac Cardiovasc Surg. 1967 May;53(5):698–706. [PubMed] [Google Scholar]
- SELYE H., BAJUSZ E., GRASSO S., MENDELL P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960 Oct;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963 Feb;12:131–155. [PubMed] [Google Scholar]