Abstract
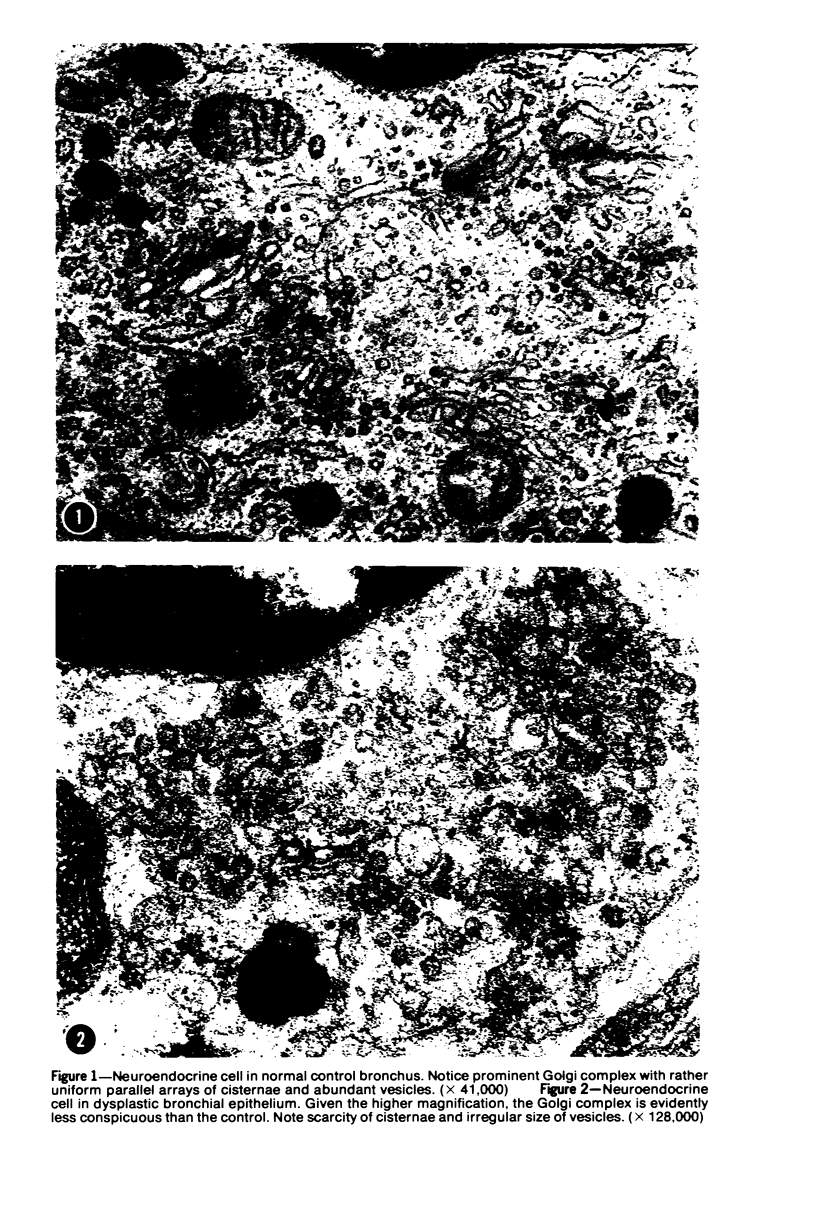
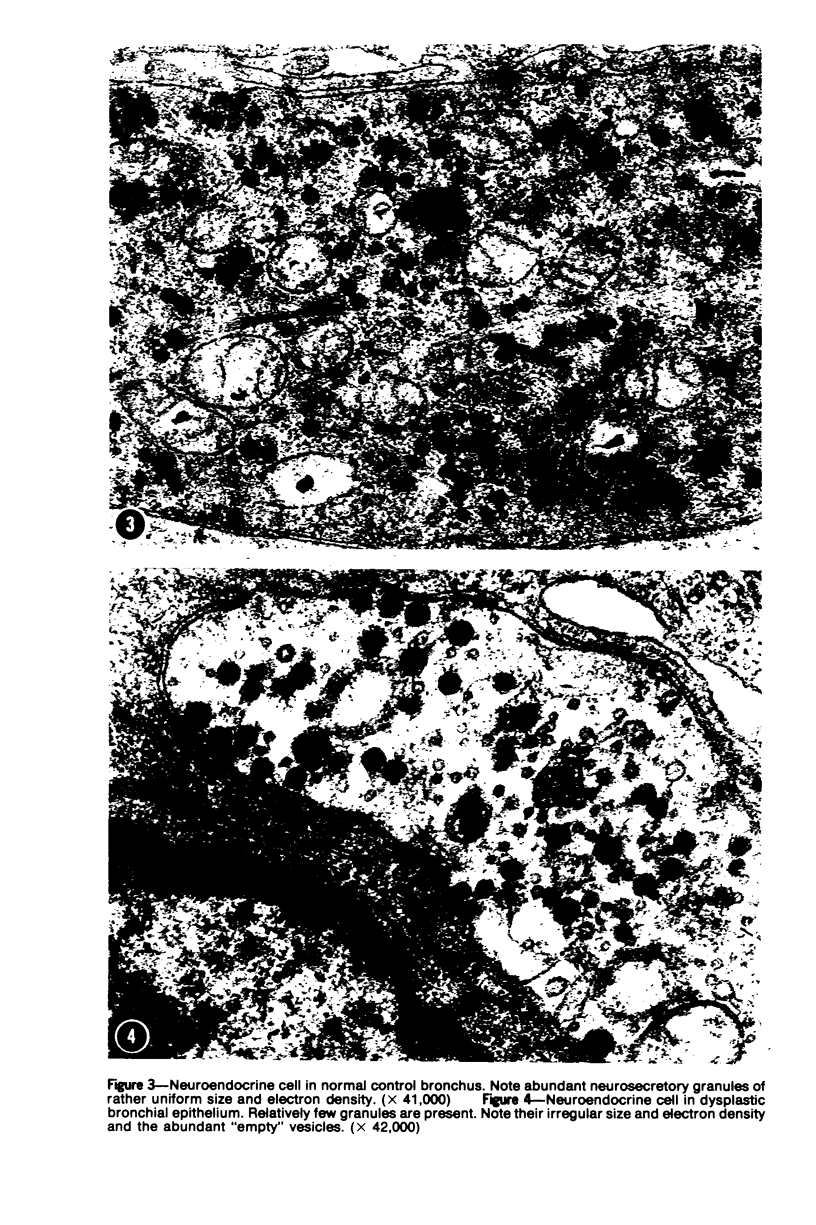
Ultrastructural and biochemical studies have suggested that bronchopulmonary carcinoids and oat cell carcinomas may be derivatives of neuroendocrine cells; their amine and/or peptide secretory capabilities may at times be reflected in clinical hormonal syndromes. This investigation was prompted by the hypothesis that dysplastic neuroendocrine bronchial cells may also exhibit structural and functional aberrations of their secretory apparatus. Surgical specimen samples from 5 human dysplastic bronchi were studied ultrastructurally; 7 normal bronchi served as controls. Golgi complexes of dysplastic cells were distinctly less prominent than those of the controls. Moreover, the Golgi vesicles of dysplastic cells appeared significantly smaller than their counterparts in normal cells (P less than 0.01). Also, dysplastic neuroendocrine cells displayed significantly fewer secretory granules per cell than the controls (P less than 0.05). These findings indicate structural abnormalities in the secretory apparatus of neuroendocrine cells in dysplastic bronchi and correlate with experimental observations of aberrant hormonal production associated with bronchial dysplasia. Thus, the possibility arises that bronchial epithelial dysplasias may be detected and monitored through laboratory determinations of their secretory products.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensch K. G., Corrin B., Pariente R., Spencer H. Oat-cell carcinoma of the lung. Its origin and relationship to bronchial carcinoid. Cancer. 1968 Dec;22(6):1163–1172. doi: 10.1002/1097-0142(196811)22:6<1163::aid-cncr2820220612>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Gewirtz G., Schneider B., Krieger D. T., Yalow R. S. Big ACTH: conversion to biologically active ACTH by trypsin. J Clin Endocrinol Metab. 1974 Feb;38(2):227–230. doi: 10.1210/jcem-38-2-227. [DOI] [PubMed] [Google Scholar]
- Gewirtz G., Yalow R. S. Ectopic ACTH production in carcinoma of the lung. J Clin Invest. 1974 Apr;53(4):1022–1032. doi: 10.1172/JCI107639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould V. E., Benditt E. P. Ultrastructural and functional relationships of some human endocrine tumors. Pathol Annu. 1973;8:205–230. [PubMed] [Google Scholar]
- Gregory R. A., Tracy H. J. Isolation of two "big gastrins" from Zollinger-Ellison tumour tissue. Lancet. 1972 Oct 14;2(7781):797–799. doi: 10.1016/s0140-6736(72)92151-4. [DOI] [PubMed] [Google Scholar]
- Hammar S., Sale G. Multiple hormone producing islet cell carcinomas of the pancreas. A morphological and biochemical investigation. Hum Pathol. 1975 May;6(3):349–362. doi: 10.1016/s0046-8177(75)80097-9. [DOI] [PubMed] [Google Scholar]
- Lacy P. E. Endocrine secretory mechanisms. A review. Am J Pathol. 1975 Apr;79(1):170–188. [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Barrett L. A., Trump B. F. Observations on small granule cells in adult human bronchial epithelium and in carcinoid and oat cell tumors. Lab Invest. 1976 Feb;34(2):202–206. [PubMed] [Google Scholar]
- Pearse A. G. The APUD cell concept and its implications in pathology. Pathol Annu. 1974;9(0):27–41. [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzakis J. A., Sommers S. C., Andersson B. Neurosecretory appearing cells of human segmental bronchi. Lab Invest. 1972 Jan;26(1):127–132. [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]