Abstract
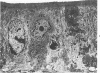
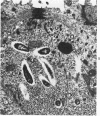
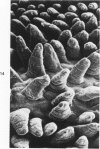
The ultrastructure of developing ileal lesions was characterized in weanling hamsters with experimentally induced transmissible ileal hyperplasia (TIH). The primary lesion was mucosal hyperplasia with progressive replacement of mature villus columnar absorptive cells by undifferentiated crypt-type cells. The undifferentiated, mitotically active cells expanded onto villus walls from their normal location in crypts by Day 10 and reached villus tips by Day 14. Aggregates of slightly curved, 0.3 X 1.5 mu, rod-shaped bacteria were detected in the apical cytoplasm of crypt epithelium by Day ;. They replicated intracellularly and accumulated in progressively greater numbers in hyperplastic cells. Active penetration of cells by intralumenal bacteria was not seen. The appearance and distribution of TIH-associated antigen, demonstrated by indirect immunofluorescence, was identical to that observed for intracellular bacteria. Hyperplastic, bacteria-laden crypt epithelium penetrated adjacent supporting tissues. Dilated crypts with flattened epithelium ruptured and released organisms into surrounding tissues. Pyogranulomatous inflammation began at 17 to 25 days and preceded or accompanied penetration of the muscle layers by expanding crypts. Macrophages and neutrophils in inflammatory lesions contained many phagocytized bacteria. In some advanced lesions mature, bacteria-free absorptive cells and goblet cells reappeared. These observations support the hypothesis that intestinal bacteria cause TIH.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS G. D., SCHNEIDER H., FORMAL S. B., SPRINZ H. CELLULAR RENEWAL AND MUCOSAL MORPHOLOGY IN EXPERIMENTAL ENTERITIS. INFECTION WITH SALMONELLA TYPHIMURIUM IN THE MOUSE. Lab Invest. 1963 Dec;12:1241–1248. [PubMed] [Google Scholar]
- BIGGERS D. C., KRAFT L. M., SPRINZ H. LETHAL INTESTINAL VIRUS INFECTION OF MICE (LIVIM). AN IMPORTANT NEW MODEL FOR STUDY OF THE RESPONSE OF THE INTESTINAL MUCOSA TO INJURY. Am J Pathol. 1964 Sep;45:413–422. [PMC free article] [PubMed] [Google Scholar]
- Boothe A. D., Cheville N. F. The pathology of proliferative ileitis of the golden syrian hamster. Pathol Vet. 1967;4(1):31–44. doi: 10.1177/030098586700400104. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjaard H., van der Meer-Fieggen W., Giesen J. Feedback control by functional villus cells on cell proliferation and maturation in intestinal epithelium. Exp Cell Res. 1972 Jul;73(1):197–207. doi: 10.1016/0014-4827(72)90120-6. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Cullen J., Dowling R. H. Intestinal structure and function after small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):731–742. doi: 10.1042/cs0430731. [DOI] [PubMed] [Google Scholar]
- Jacoby R. O., Osbaldiston G. W., Jonas A. M. Experimental transmission of atypical ileal hyperplasia of hamsters. Lab Anim Sci. 1975 Aug;25(4):465–473. [PubMed] [Google Scholar]
- Jacoby R. O. Transmissible ileal hyperplasia of hamsters. I. Histogenesis and immunocytochemistry. Am J Pathol. 1978 Jun;91(3):433–450. [PMC free article] [PubMed] [Google Scholar]
- Jonas A. M., Tomita Y., Wyand D. S. Enzootic intestinal adenocarcinoma in hamsters. J Am Vet Med Assoc. 1965 Nov 15;147(10):1102–1108. [PubMed] [Google Scholar]
- Lawson G. H., Rowland A. C., Wooding P. The characterisation of Campylobacter sputorum subspecies mucosalis isolated from pigs. Res Vet Sci. 1975 Mar;18(2):121–126. [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- PADYKULA H. A. Recent functional interpretations of intestinal morphology. Fed Proc. 1962 Nov-Dec;21:873–879. [PubMed] [Google Scholar]
- Perera D. R., Weinstein W. M., Rubin C. E. Symposium on pathology of the gastrointestinal tract-Part II. Small intestinal biopsy. Hum Pathol. 1975 Mar;6(2):157–217. doi: 10.1016/s0046-8177(75)80176-6. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Rijke R. P., Plaisier H., Hoogeveen A. T., Lamerton L. F., Galjaard H. The effect of continuous irradiation on cell proliferation and maturation in small intestinal epithelium. Cell Tissue Kinet. 1975 Sep;8(5):441–453. doi: 10.1111/j.1365-2184.1975.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Rijke R. P., van der Meer-Fieggen W., Galjaard H. Effect of villus length on cell proliferation and migration in small intestinal epithelium. Cell Tissue Kinet. 1974 Nov;7(6):577–586. doi: 10.1111/j.1365-2184.1974.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Rowland A. C., Lawson G. H. Intestinal adenomatosis in the pig: a possible relationship with a haemorrhagic enteropathy. Res Vet Sci. 1975 May;18(3):263–268. [PubMed] [Google Scholar]
- Rowland A. C., Lawson G. H. Intestinal adenomatosis in the pig: immunofluorescent and electron microscopic studies. Res Vet Sci. 1974 Nov;17(3):323–330. [PubMed] [Google Scholar]
- Staley T. E., Jones E. W., Corley L. D. Attachment and penetration of Escherichia coli into intestinal epithelium of the ileum in newborn pigs. Am J Pathol. 1969 Sep;56(3):371–392. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Formal S. B., Sprinz H. Exerimental acute colitis in the Rhesus monkey following peroral infection with Shigella flexneri. An electron microscope study. Am J Pathol. 1968 Mar;52(3):503–529. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H., LaBrec E. H., Formal S. B. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am J Pathol. 1965 Dec;47(6):1011–1044. [PMC free article] [PubMed] [Google Scholar]
- Tilson M. D., Wright H. K. Adaptation of functioning and bypassed segments of ileum during compensatory hypertrophy of the gut. Surgery. 1970 Apr;67(4):687–693. [PubMed] [Google Scholar]
- Tutton P. J. Control of epithelial cell proliferation in the small intestinal crypt. Cell Tissue Kinet. 1973 May;6(3):211–216. doi: 10.1111/j.1365-2184.1973.tb01610.x. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. E., Owens D. R., Troutt H. F. Proliferative ileitis of hamsters: electron microscopy of bacteria in cells. Am J Vet Res. 1973 Feb;34(2):249–252. [PubMed] [Google Scholar]
- Withers H. R. Regeneration of intestinal mucosa after irradiation. Cancer. 1971 Jul;28(1):75–81. doi: 10.1002/1097-0142(197107)28:1<75::aid-cncr2820280115>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]