Abstract
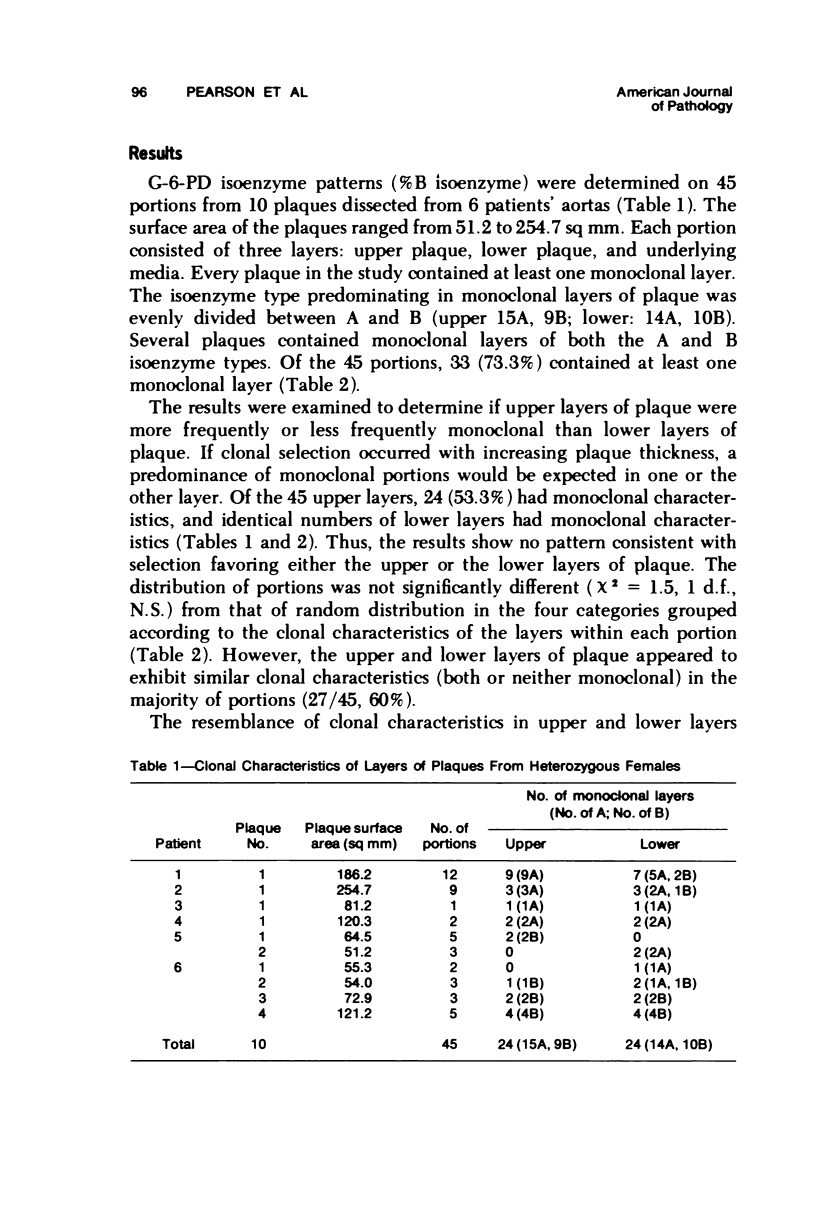
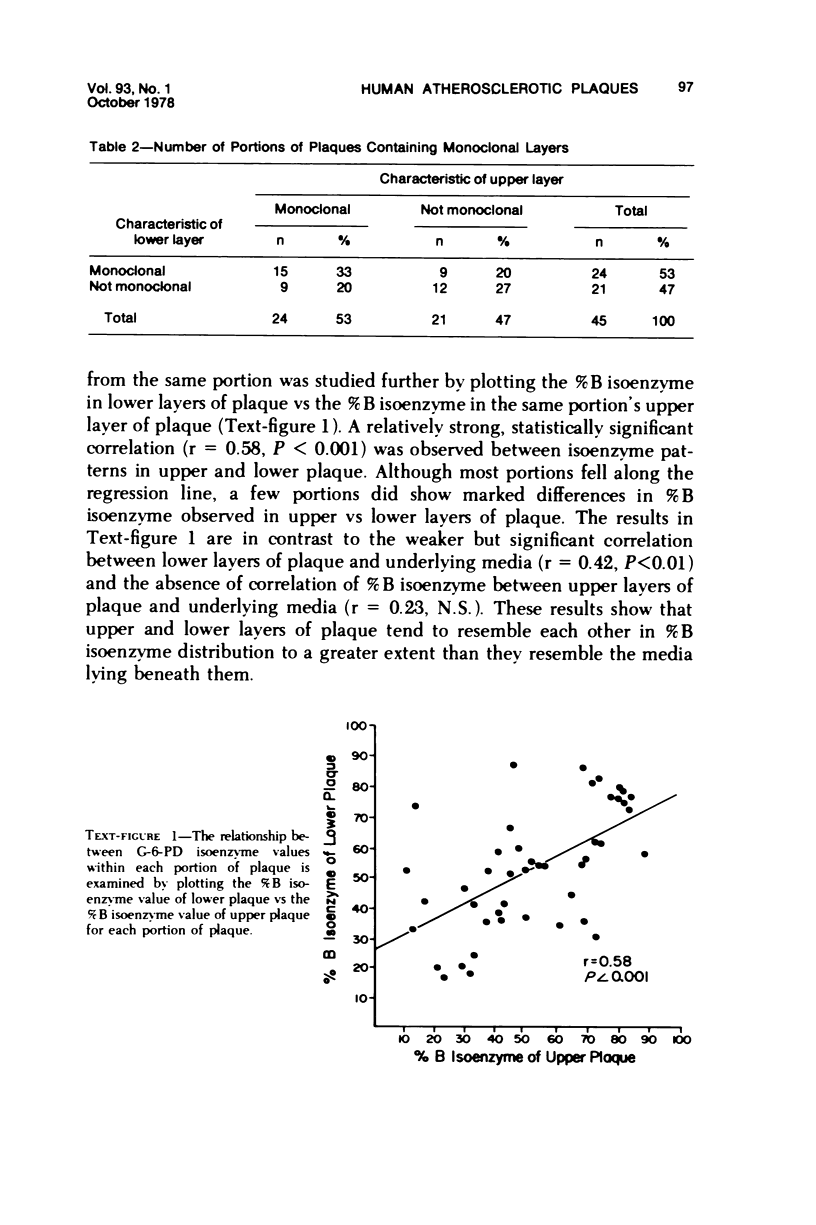
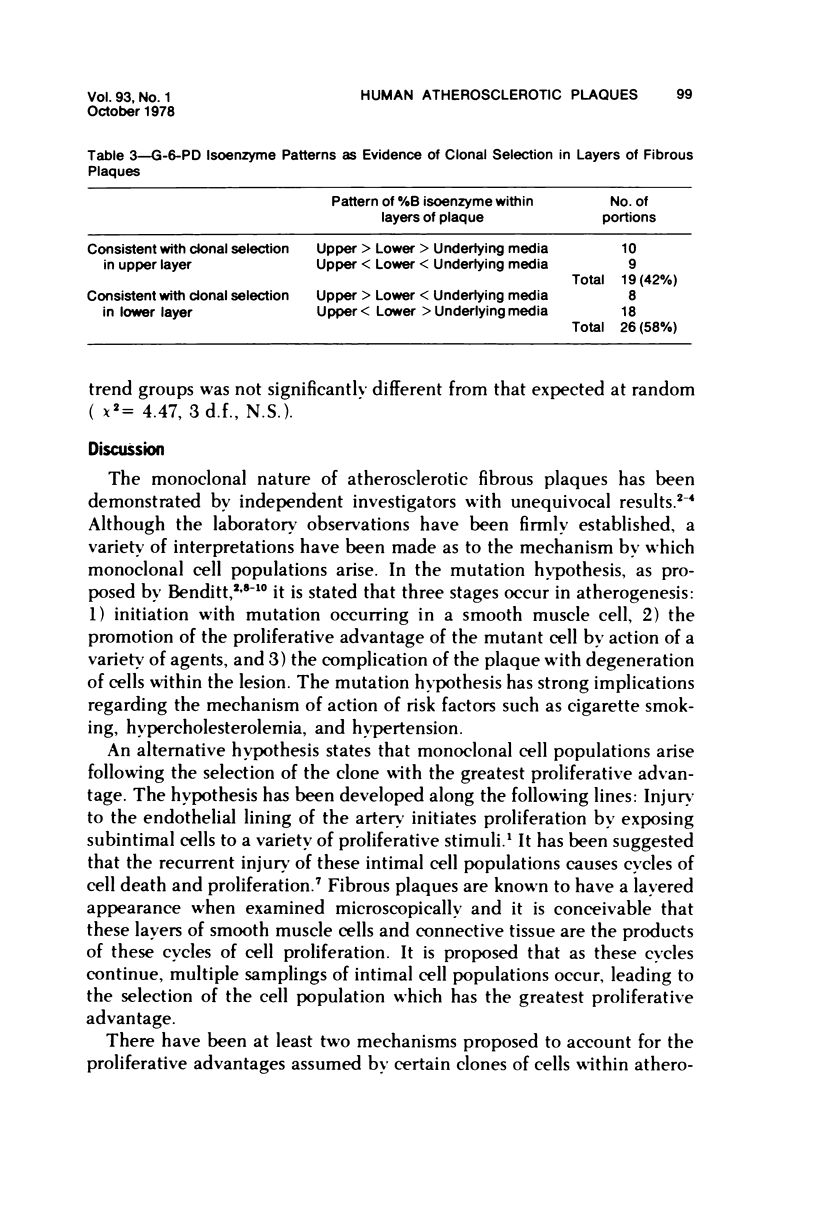
Two hypotheses have been proposed to explain the origin of monoclonal cell populations within human atherosclerotic plaques. The first of these proposes a mutational origin: the second suggests that the single clone of cells with the greatest proliferative advantage is selected following repetitive intimal injury. If the selection hypothesis is true, monoclonality should be observed more frequently in the layer of plaque most recently formed. Glucose-6-phosphate dehydrogenase (G-6-PD) isoenzymes were used as cellular markers in aortas of females heterozygous for the A and B isoenzymes. Ten plaques were divided into 45 portions, each of which was subdivided into upper layer, lower layer, and underlying media. No predominance of monoclonality was observed in the upper or lower layers of plaque, with 53% of samples from each layer being monoclonal. In all, 73% of portions of plaque contained at least one monoclonal layer. The layers tended to resemble each other in their clonal characteristics, with 60% of portions having layers with the same clonal characteristics. A significant correlation between isoenzyme distributions in upper and lower layers of the same portion was observed. No consistent trends in isoenzyme distribution in the three layers of each portion were observed. The results are interpreted as providing no evidence for clonal selection as the mechanism by which human atherosclerotic plaques become monoclonal.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P. Evidence for a monoclonal origin of human atherosclerotic plaques and some implications. Circulation. 1974 Oct;50(4):650–652. doi: 10.1161/01.cir.50.4.650. [DOI] [PubMed] [Google Scholar]
- Benditt E. P. Implications of the monoclonal character of human atherosclerotic plaques. Beitr Pathol. 1976 Sep;158(4):405–416. doi: 10.1016/s0005-8165(76)80137-0. [DOI] [PubMed] [Google Scholar]
- Benditt E. P. The origin of atherosclerosis. Sci Am. 1977 Feb;236(2):74–85. doi: 10.1038/scientificamerican0277-74. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. The origin and development of human tumors studied with cell markers. N Engl J Med. 1974 Jul 4;291(1):26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- GARTLER S. M., LINDER D. SELECTION IN MAMMALIAN MOSAIC CELL POPULATIONS. Cold Spring Harb Symp Quant Biol. 1964;29:253–260. doi: 10.1101/sqb.1964.029.01.028. [DOI] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Linder D., Gartler S. M. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965 Oct 1;150(3692):67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Norwood T. H., Pendergrass W. R. Clonal selection, attenuation and differentiation in an in vitro model of hyperplasia. Am J Pathol. 1974 Jan;74(1):137–154. [PMC free article] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A. Symposium on in vitro studies related to atherogenesis. Life histories of hyperplastoid cell lines from aorta and skin. Exp Mol Pathol. 1973 Apr;18(2):125–141. doi: 10.1016/0014-4800(73)90012-9. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Kramer E. C., Solez K., Heptinstall R. H. The human atherosclerotic plaque. Am J Pathol. 1977 Mar;86(3):657–664. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Wang B. A., Solez K., Heptinstall R. H. Clonal characteristics of fibrous plaques and fatty streaks from human aortas. Am J Pathol. 1975 Nov;81(2):379–387. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Florentin R. A., Reiner J. M., Lee W. M., Lee K. T. Alterations in population dynamics of arterial smooth muscle cells during atherogenesis. IV. Evidence for a polyclonal origin of hypercholesterolemic diet-induced atherosclerotic lesions in young swine. Exp Mol Pathol. 1976 Apr;24(2):244–260. doi: 10.1016/0014-4800(76)90009-5. [DOI] [PubMed] [Google Scholar]



