Abstract
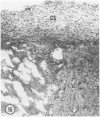
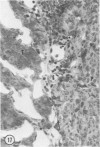
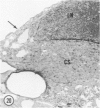
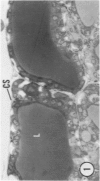
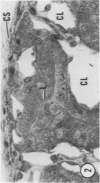
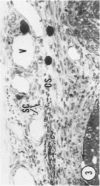
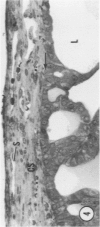
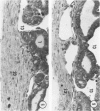
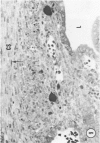
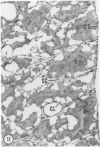
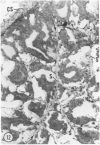
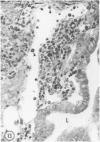
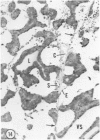
Young adult male Fischer rats were fed 0.25% thouracil in a low-iodine diet to produce hyperplasia of the thyroid gland. The capsule of the thyroid gland increased in thickness from approximately one cell in controls to a substantial multilayered structure. Increase in capsule thickness was noted by 3 days. The cell population of the capsule was largely fibroblasts, but during a period within the interval from 14 to 28 days, the capsule tended to be exceptionally thick and contained many mononuclear leukocytes. At later times the capsule was not quite as thick and the leukocytes largely disapproved. Capillaries developed in the capsule probably by sprouting. The capsule growth was so extensive that certain neighboring tissues were often incorporated into the capsule, including arteries, veins, nerves, striated muscle, and lymph nodes. There was some regional specificity in the development of capsular hyperplasia. Connective tissue increased around the thyroid and parathyroid glands but not between them. Connective tissue in partitions with the thyroid gland also increased in thickness, although the extent of accumulation of cells and intercellular matrix was much less than in the capsule.
Full text
PDF









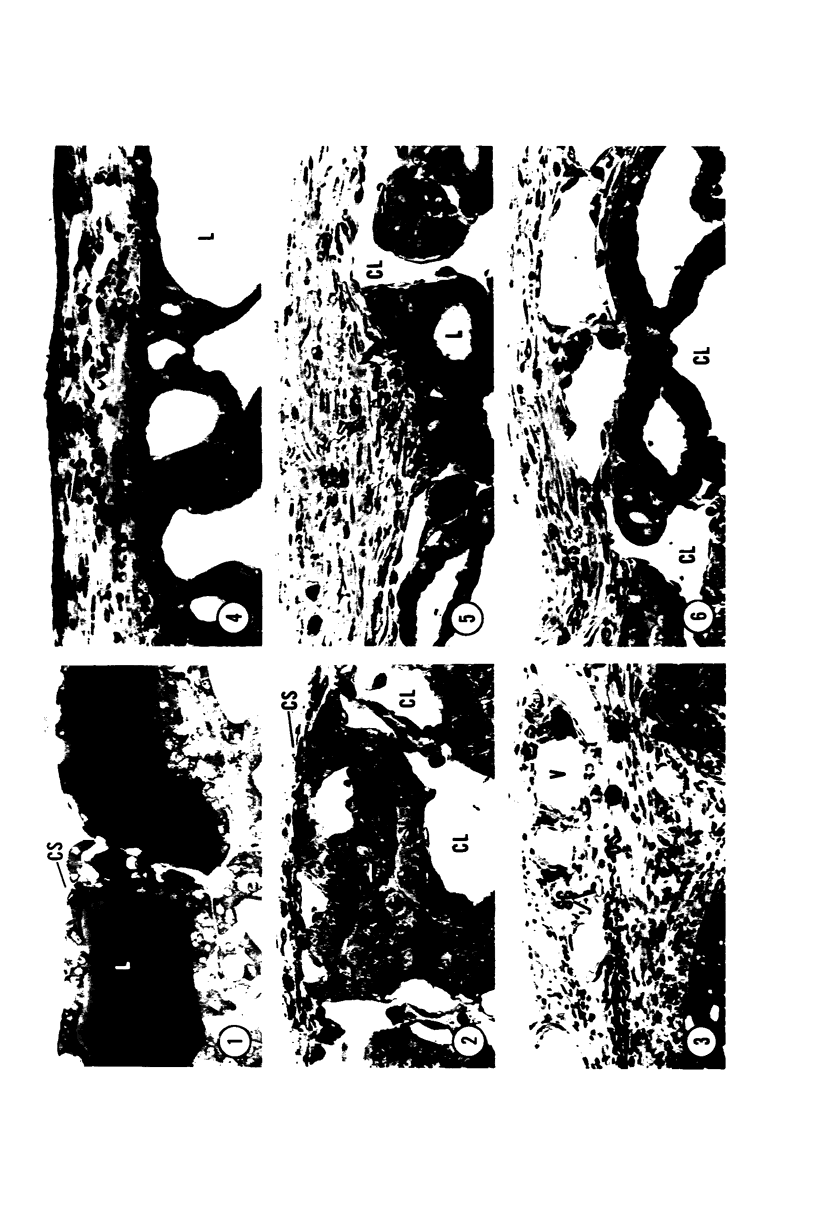
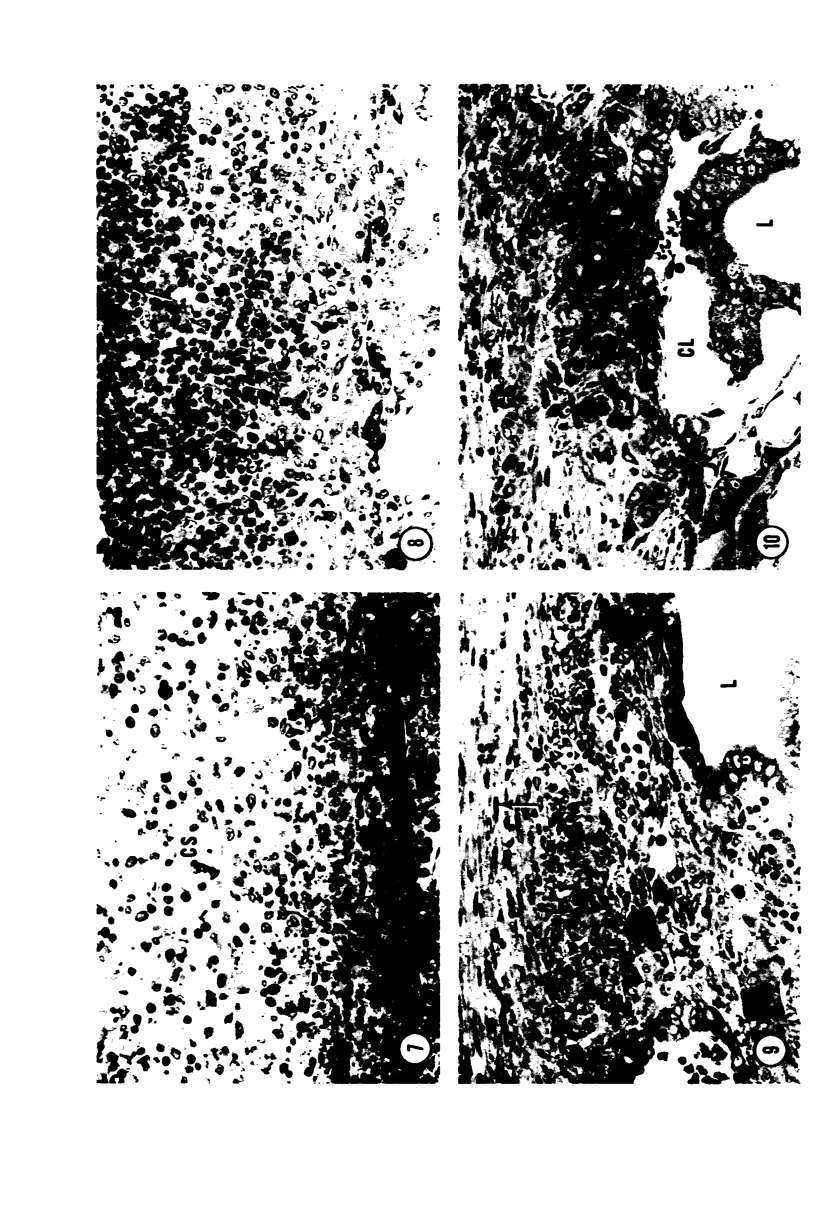
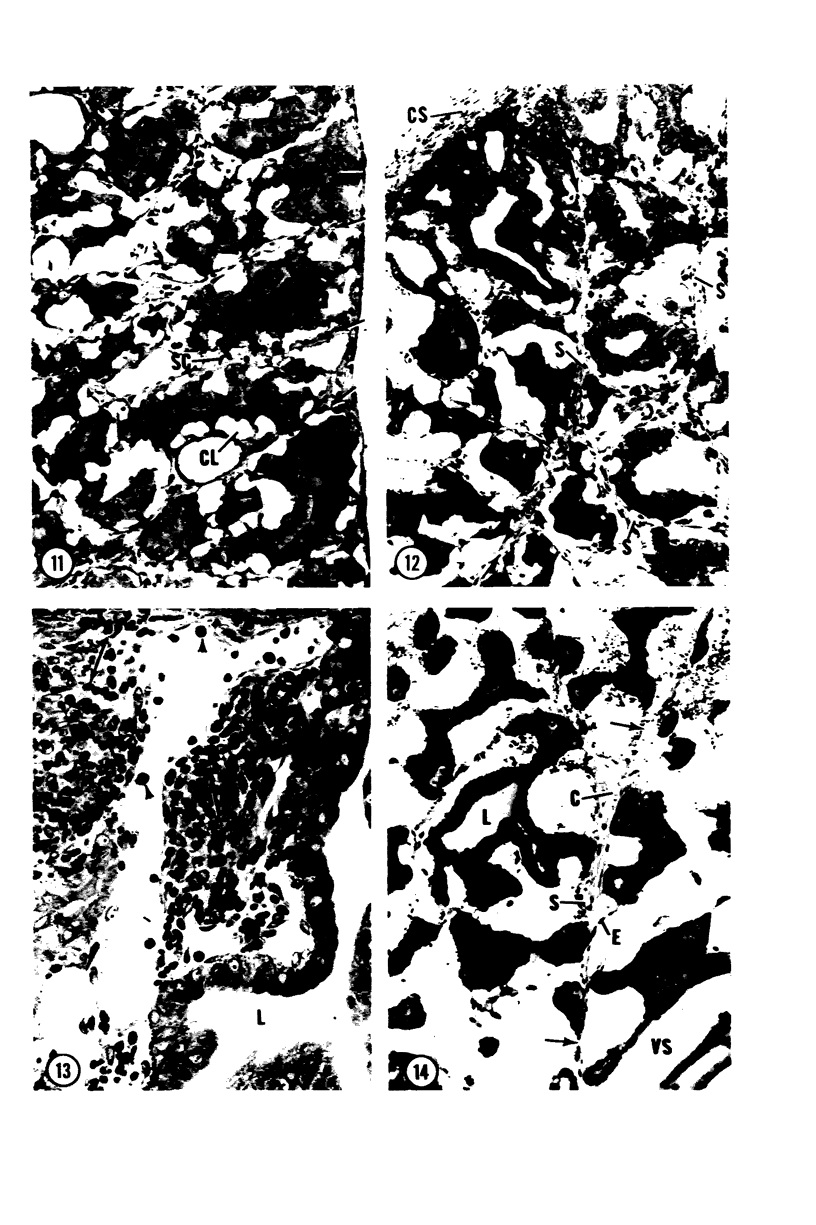


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISCHOFF F., BRYSON G. CARCINOGENESIS THROUGH SOLID STATE SURFACES. Prog Exp Tumor Res. 1964;5:85–133. doi: 10.1159/000385997. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976 Jun 1;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Seyer J. M., Kang A. H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci U S A. 1978 Feb;75(2):871–875. doi: 10.1073/pnas.75.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Everett N. B., Tyler R. Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. J Cell Biol. 1970 Mar;44(3):645–654. doi: 10.1083/jcb.44.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASILIEV J. M., OLSHEVSKAJA L. V., RAIKHLIN N. T., IVANOVA O. J. Comparative study of alterations induced by 7,12-dimethylbenz[a]anthracene and polymer films in the subcutaneous connective tissue of rats. J Natl Cancer Inst. 1962 Mar;28:515–559. [PubMed] [Google Scholar]
- Wollman S. H., Herveg J. P., Zeligs J. D., Ericson L. E. Blood capillary enlargement during the development of thyroid hyperplasia in the rat. Endocrinology. 1978 Dec;103(6):2306–2314. doi: 10.1210/endo-103-6-2306. [DOI] [PubMed] [Google Scholar]
- Zeilgs J. D., Wollman S. H. Microhemorrhage in the hyperplastic thyroid gland of the rat. Am J Pathol. 1976 Nov;85(2):317–332. [PMC free article] [PubMed] [Google Scholar]