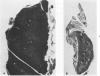
Abstract
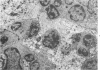
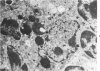
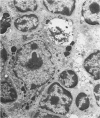
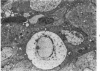
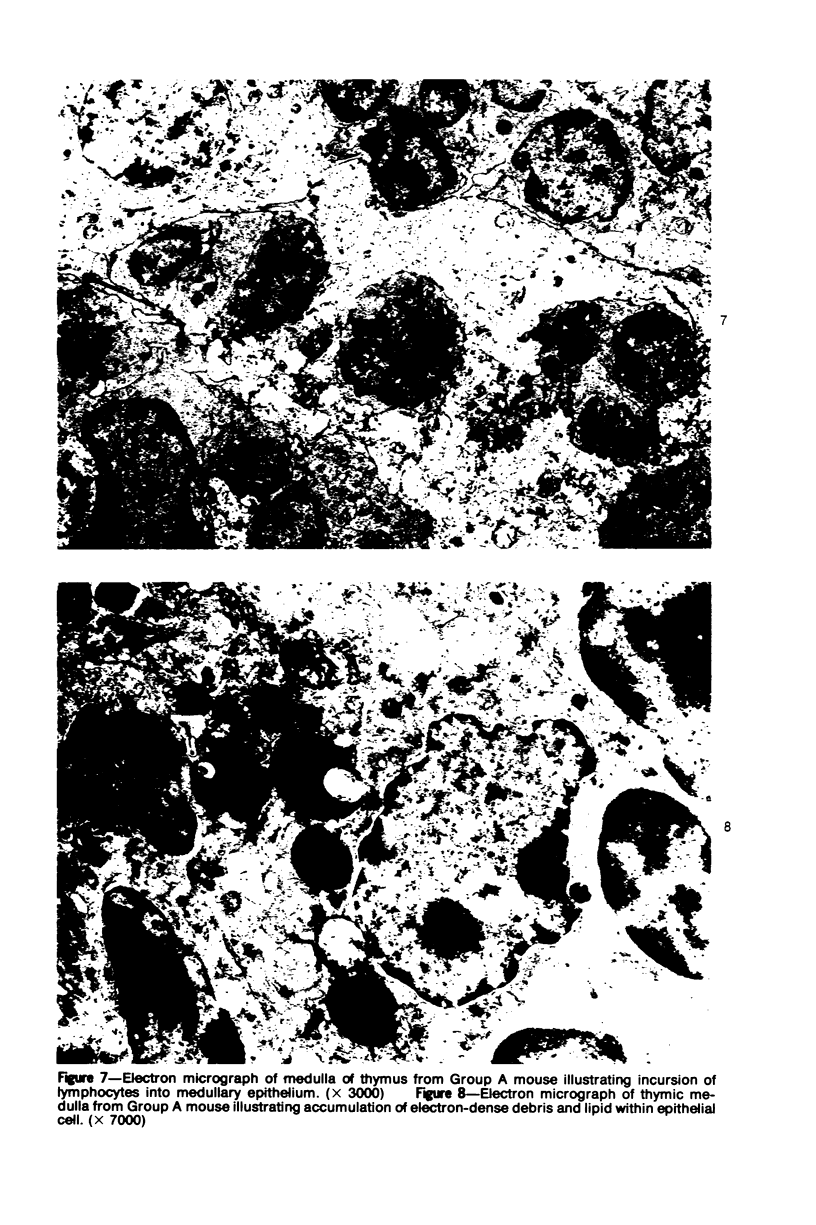
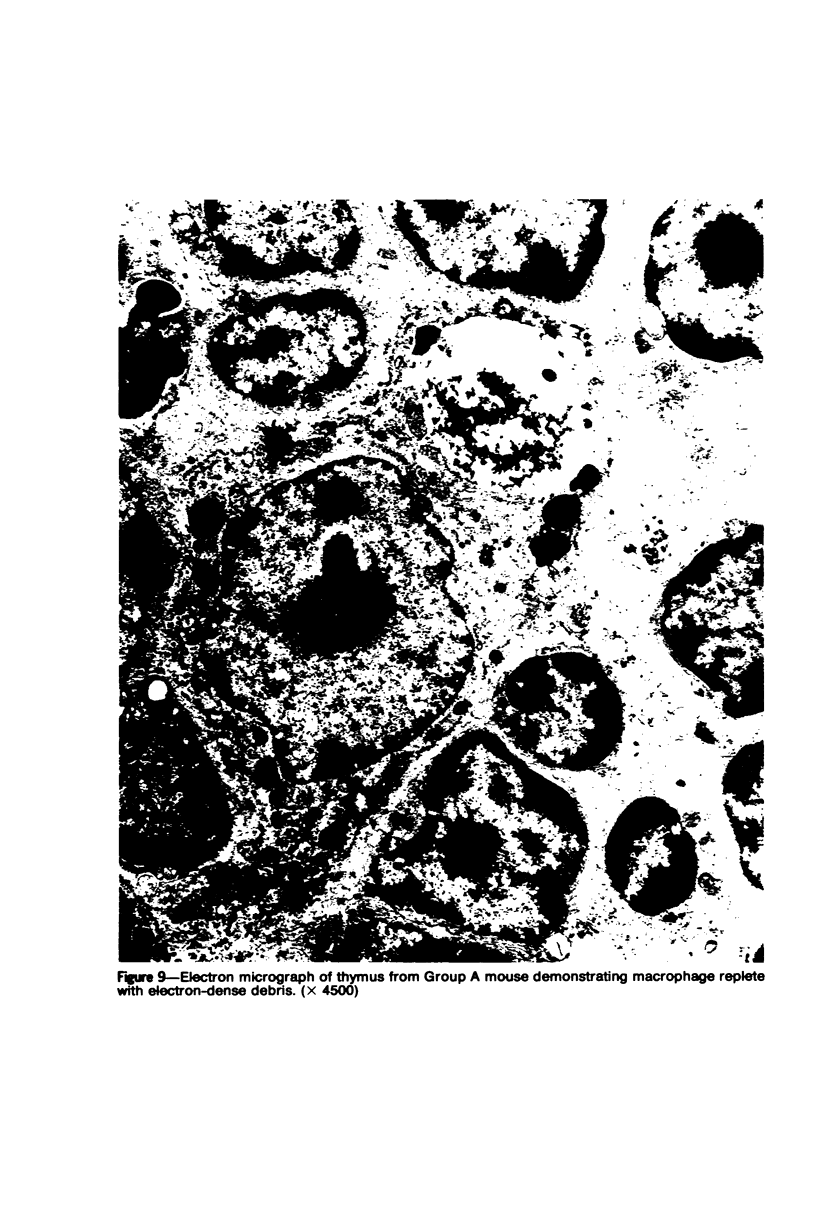
In four separate experiments 140 adults A(H-2a) x C57BL/6(H-2b) F1hybrid mice were surgically adrenalectomized and divided into three experimental groups. Seventy-one additional adult F1hybrids (AXC57BL/6) which had not been adrenalectomized were divided into three similar groups. In Group 1 (GvH group), GvH reactions were induced by the injection of 50 x 106 pooled parental lymphoid cells intravenously. The second group (syngeneic group) received 50 x 106 pooled F1 hybrid lymphoid cells intravenously. The third group (uninoculated group) received no lymphoid inoculum. At regular intervals the animals were killed, autopsied, and histologically studied. Visceral alterations of GvH reaction were recorded in the thymus, lymph nodes, spleen, and liver in the GvH groups; none was present in the other groups. The thymuses in the nonadrenalectomized GvH group underwent prompt involution characterized by size reduction and cortical lymphoid cell depletion. These changes were not apparent in the GvH adrenalectomized group. Both GvH groups, however, demonstrated an effacement of the medulla, lymphocyte incursion into the medulla, lymphocyte emperipolesis of medullary epithelial cells, gradual disappearance of Hassall's corpuscles, epithelial cell injury, and an ingress of macrophages laden with nuclear and cellular debris. This study suggests that the stress and corticosteroid response which accompany a GvH reaction account for the reduction in the thymic size and cortical lymphoid cell mass. The medullary alterations, therefore, would appear to be initiated by the GvH reaction per se.
Full text
PDF







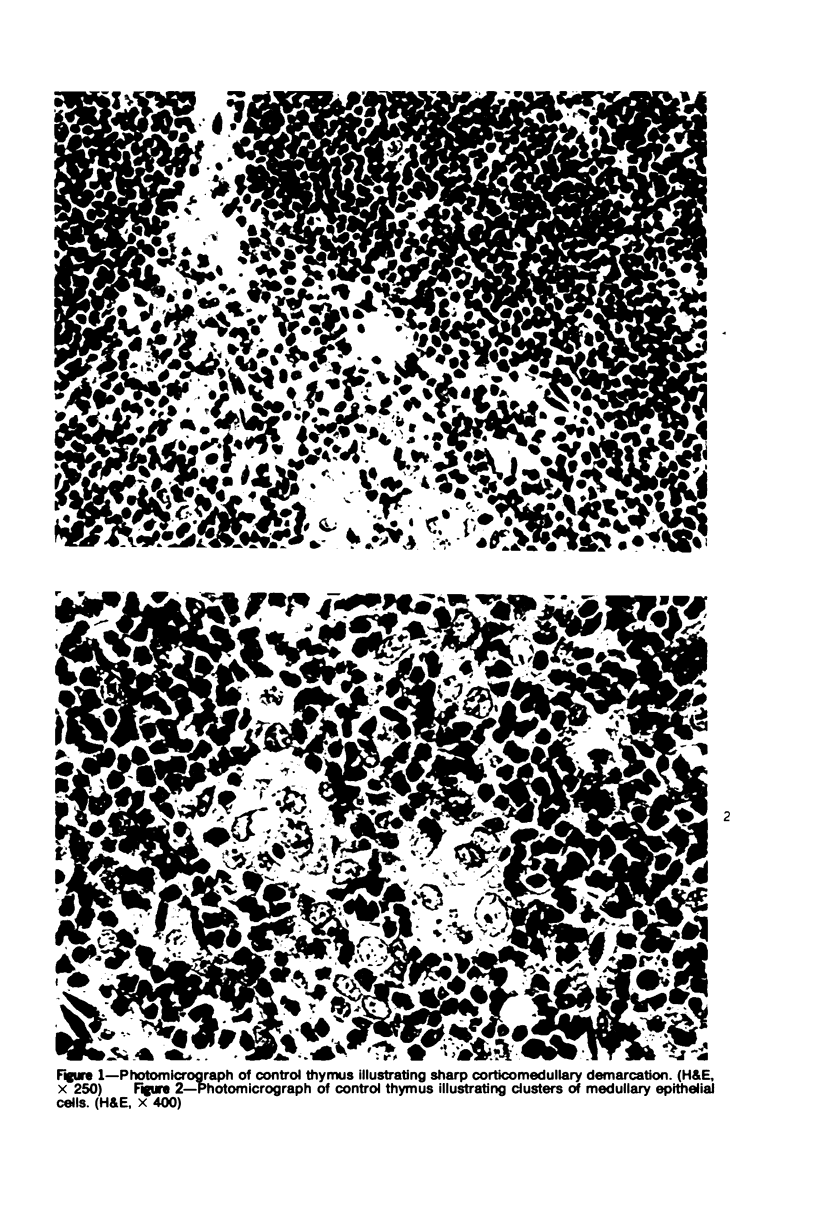

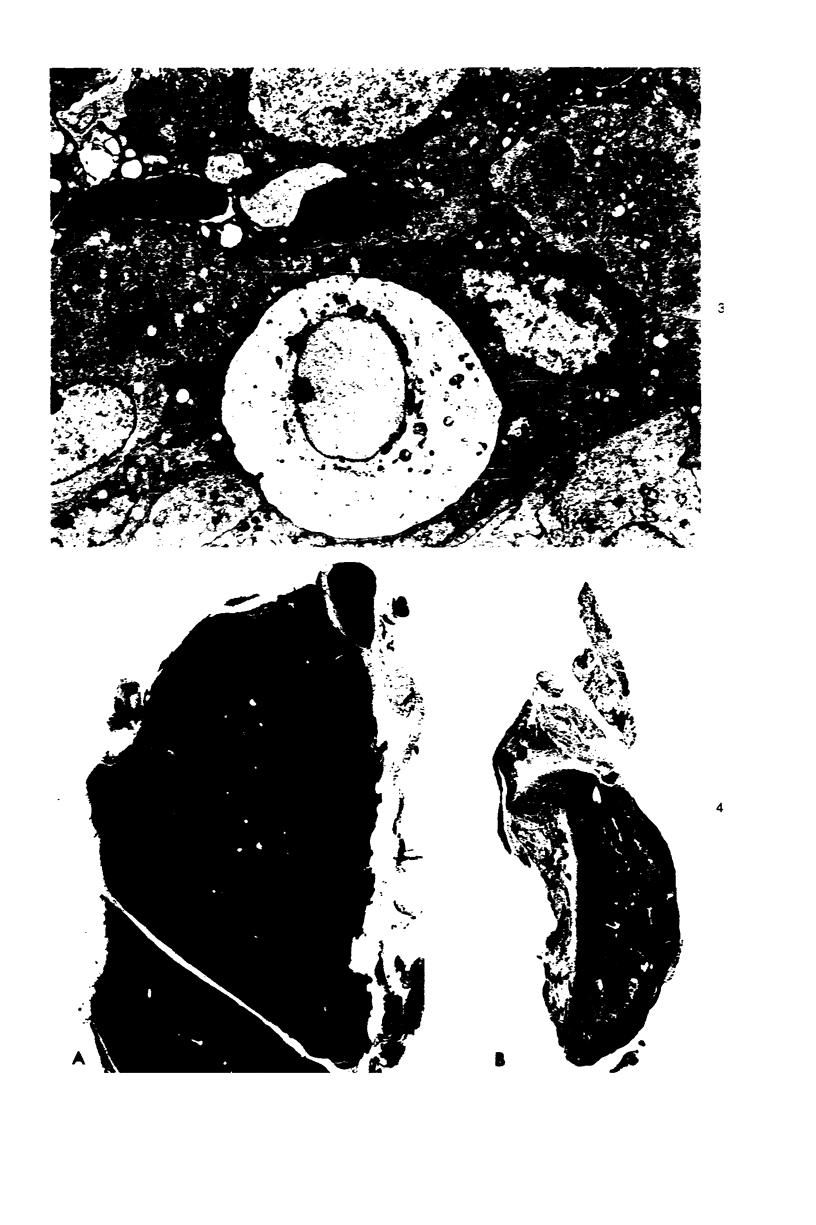
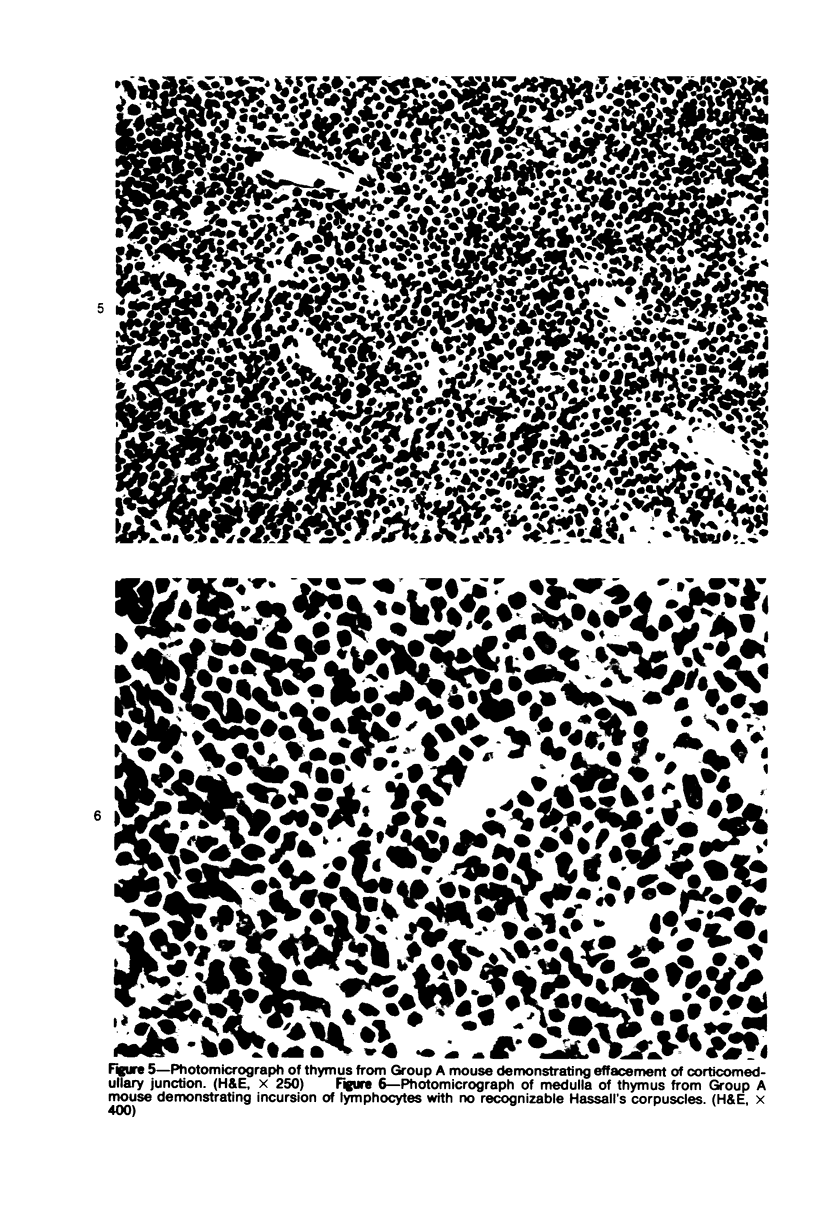
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elie R., Lapp W. S. Graft-versus-host induced immunosuppression: depressed T cell helper function in vitro. Cell Immunol. 1976 Jan;21(1):31–39. doi: 10.1016/0008-8749(76)90324-5. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Grushka M., Lapp W. S. Effect of lymphoid tissue transplants on the intensity of an existing graft-versus-host reaction. Transplantation. 1974 Feb;17(2):157–163. doi: 10.1097/00007890-197402000-00001. [DOI] [PubMed] [Google Scholar]
- HARRIS J. E., FORD C. E. CELLULAR TRAFFIC OF THE THYMUS: EXPERIMENTS WITH CHROMOSOME MARKERS. EVIDENCE THAT THE THYMUS PLAYS AN INSTRUCTIONAL PART. Nature. 1964 Feb 29;201:884–885. doi: 10.1038/201884a0. [DOI] [PubMed] [Google Scholar]
- Heim L. R., Martinez C., Good R. A. Cause of homologous disease. Nature. 1967 Apr 1;214(5083):26–29. doi: 10.1038/214026a0. [DOI] [PubMed] [Google Scholar]
- Heim L. R., Yunis E. J., Good R. A. Pathogenesis of graft-versus-host reaction. I. Influence of thymectomy and adrenalectomy on development of lymphopenia. Proc Soc Exp Biol Med. 1972 Mar;139(3):793–798. doi: 10.3181/00379727-139-36239. [DOI] [PubMed] [Google Scholar]
- Lapp W. S., Möller G. Prolonged survival of H-2 incompatible skin allografts on F1 animals treated with parental lymphoid cells. Immunology. 1969 Sep;17(3):339–344. [PMC free article] [PubMed] [Google Scholar]
- Lapp W. S., Wechsler A., Kongshavn P. A. Immune restoration of mice immunosuppressed by a graft-vs.-host reaction. Cell Immunol. 1974 Mar 30;11(1-3):419–426. doi: 10.1016/0008-8749(74)90040-9. [DOI] [PubMed] [Google Scholar]
- Miller J. F. Editorial: Endocrine function of the thymus. N Engl J Med. 1974 May 30;290(22):1255–1256. doi: 10.1056/NEJM197405302902211. [DOI] [PubMed] [Google Scholar]
- Pickel K., Hoffmann M. K. The Ly phenotype of suppressor T cells arising in mice subjected to a graft-versus-host reaction. J Exp Med. 1977 May 1;145(5):1169–1175. doi: 10.1084/jem.145.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potworowski E. F., Borduas A. G., Lussier G. Specific antigenicity of thymic reticuloepithelial cells. J Immunol. 1973 Oct;111(4):1292–1294. [PubMed] [Google Scholar]
- SIMONSEN M. Graft versus host reactions. Their natural history, and applicability as tools of research. Prog Allergy. 1962;6:349–467. [PubMed] [Google Scholar]
- Seemayer T. A., Lapp W. S., Bolande R. P. Thymic involution in murine graft-versus-host reaction. Epithelial injury mimicking human thymic dysplasia. Am J Pathol. 1977 Jul;88(1):119–134. [PMC free article] [PubMed] [Google Scholar]
- Shand F. L. Analysis of immunosuppression generated by the graft-versus-host reaction. I. A suppressor T-cell component studied in vivo. Immunology. 1975 Dec;29(6):953–965. [PMC free article] [PubMed] [Google Scholar]
- van Haelst U. Light and electron microscopic study of the normal and pathological thymus of the rat. II. The acute thymic involution. Z Zellforsch Mikrosk Anat. 1967;80(2):153–182. doi: 10.1007/BF00337454. [DOI] [PubMed] [Google Scholar]