Abstract
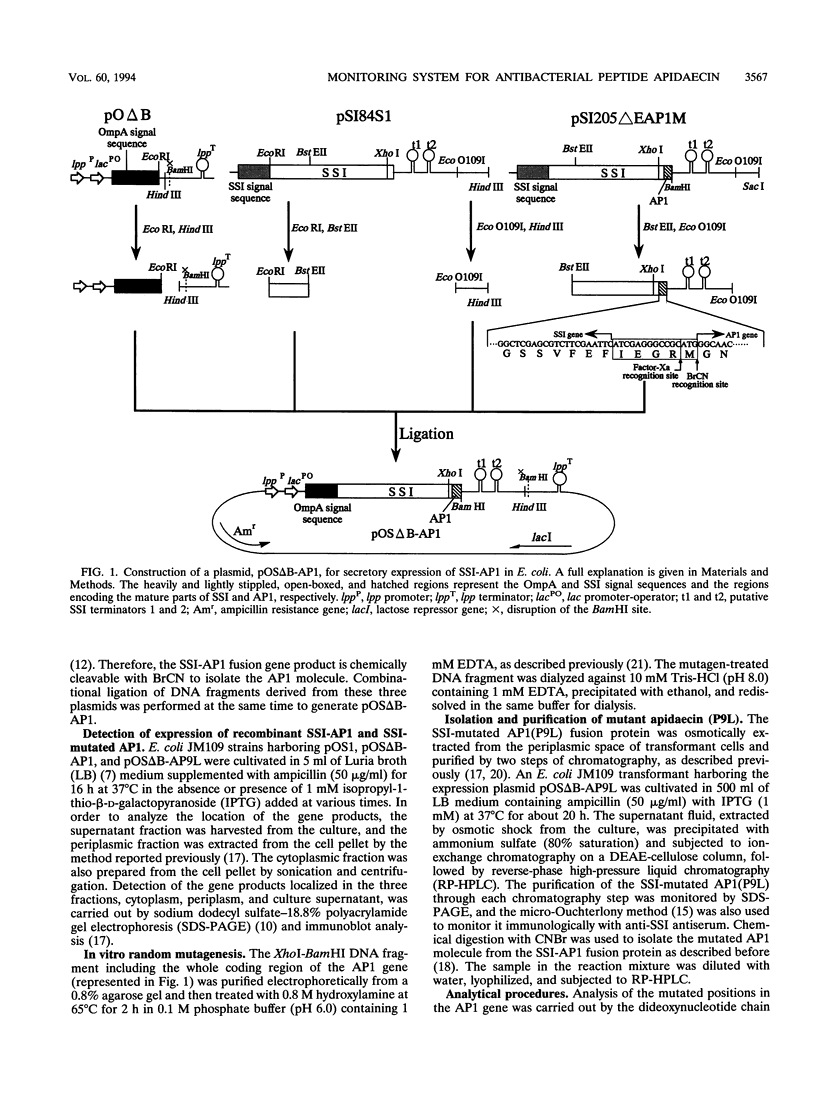
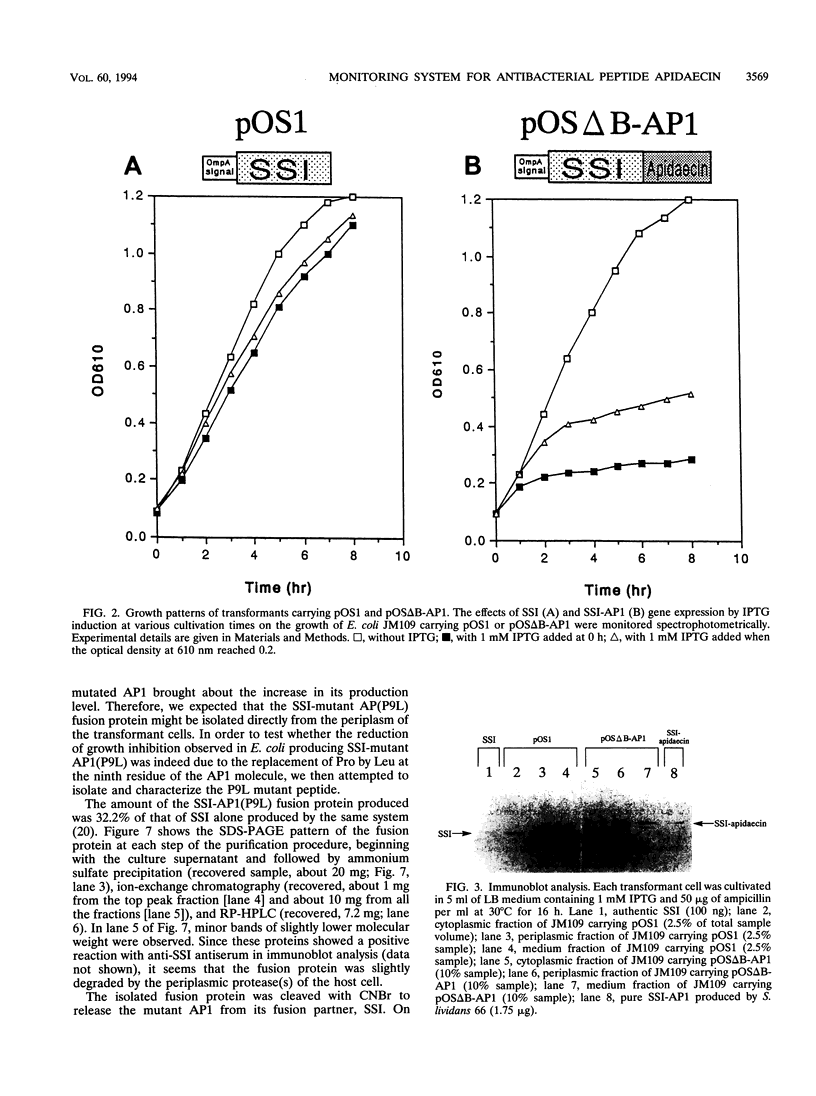
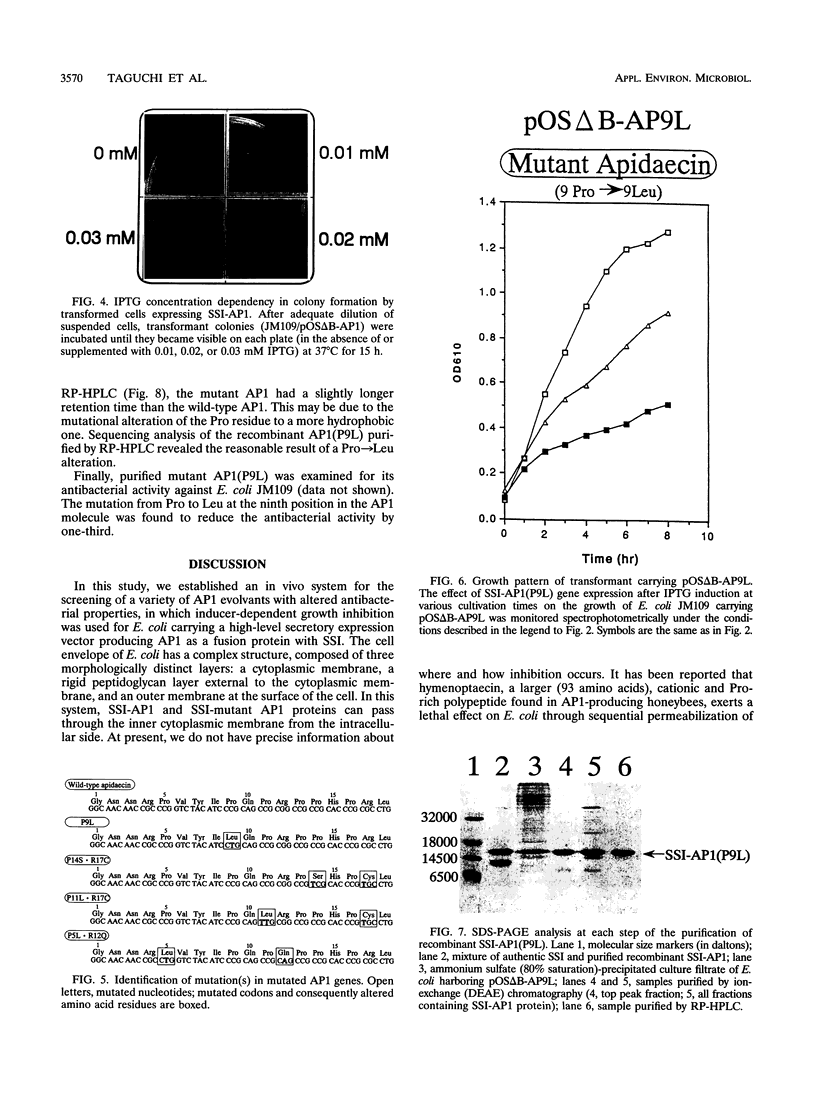
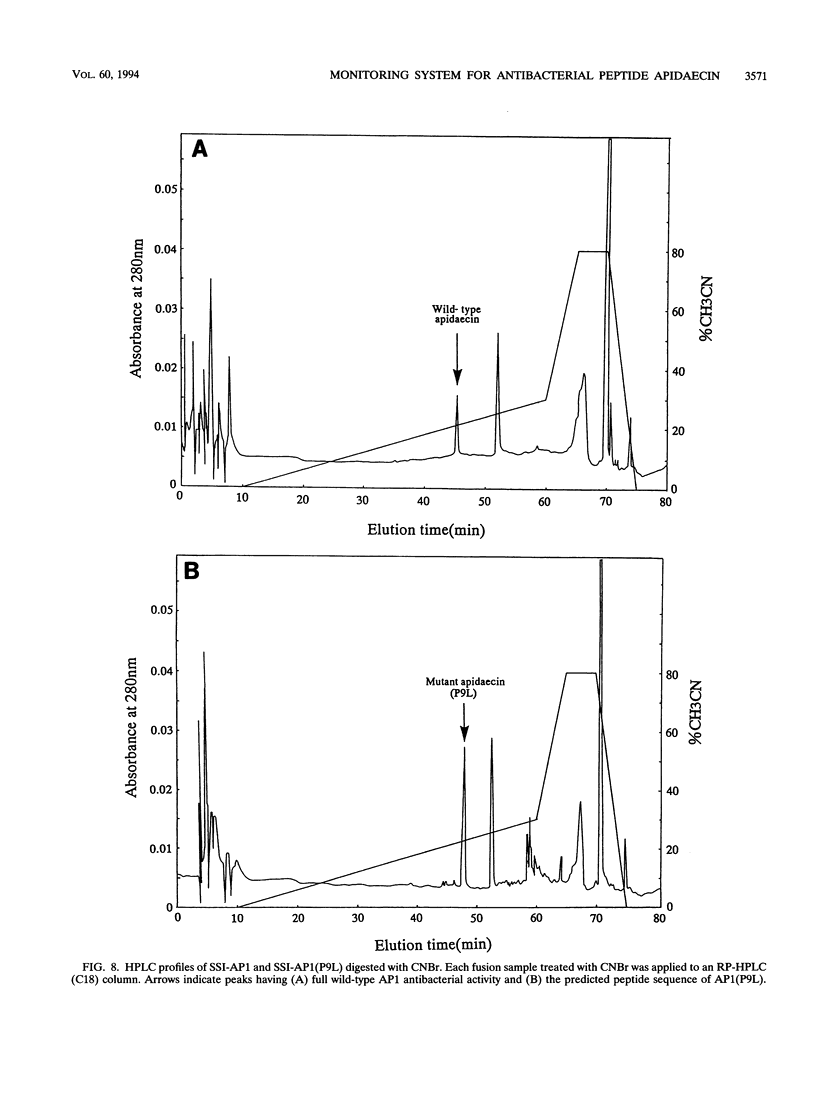
A unique antibacterial peptide derivative found in immune honeybee lymph, apidaecin 1b (AP1), was randomly mutagenized and characterized by a newly established system to analyze in vivo its structure-function relationship. Initially, a high-level expression host-vector system for AP1 in Escherichia coli was constructed by creating a fusion protein with the highly stable Streptomyces subtilisin inhibitor (SSI) molecule. Expression of the SSI-AP1 fusion protein was found to depend on the concentration of the transcriptional inducer isopropyl-beta-D-thio-galactopyranoside (IPTG) and to parallel the degree of growth inhibition of the transformant cells. Subsequently, apidaecin derivatives produced by localized random mutagenesis were screened with this IPTG concentration-controlled in vivo system by monitoring the growth inhibition patterns of the transformant cells. One mutant apidaecin (P9L) that had reduced activity was purified and isolated from the periplasmic fraction of an E. coli transformant. Its antibacterial activity was reduced to one-third of that of wild-type apidaecin. When considered together with the other mutations, it was concluded that several Pro residues, including that at the ninth position, are responsible for expression of the antibacterial action of apidaecin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Casteels-Josson K., Capaci T., Casteels P., Tempst P. Apidaecin multipeptide precursor structure: a putative mechanism for amplification of the insect antibacterial response. EMBO J. 1993 Apr;12(4):1569–1578. doi: 10.1002/j.1460-2075.1993.tb05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels P., Ampe C., Jacobs F., Tempst P. Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J Biol Chem. 1993 Apr 5;268(10):7044–7054. [PubMed] [Google Scholar]
- Casteels P., Ampe C., Jacobs F., Vaeck M., Tempst P. Apidaecins: antibacterial peptides from honeybees. EMBO J. 1989 Aug;8(8):2387–2391. doi: 10.1002/j.1460-2075.1989.tb08368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels P., Ampe C., Riviere L., Van Damme J., Elicone C., Fleming M., Jacobs F., Tempst P. Isolation and characterization of abaecin, a major antibacterial response peptide in the honeybee (Apis mellifera). Eur J Biochem. 1990 Jan 26;187(2):381–386. doi: 10.1111/j.1432-1033.1990.tb15315.x. [DOI] [PubMed] [Google Scholar]
- Casteels P., Tempst P. Apidaecin-type peptide antibiotics function through a non-poreforming mechanism involving stereospecificity. Biochem Biophys Res Commun. 1994 Feb 28;199(1):339–345. doi: 10.1006/bbrc.1994.1234. [DOI] [PubMed] [Google Scholar]
- Ghrayeb J., Kimura H., Takahara M., Hsiung H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984 Oct;3(10):2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G., Haiml L., Suchanek G. Stepwise cleavage of the pro part of promelittin by dipeptidylpeptidase IV. Evidence for a new type of precursor--product conversion. Eur J Biochem. 1980 Oct;111(1):49–58. doi: 10.1111/j.1432-1033.1980.tb06073.x. [DOI] [PubMed] [Google Scholar]
- Kylsten P., Samakovlis C., Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990 Jan;9(1):217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeno M., Taguchi S., Momose H. Production of antibacterial peptide 'apidaecin' using the secretory expression system of Streptomyces. Biosci Biotechnol Biochem. 1993 Jul;57(7):1206–1207. doi: 10.1271/bbb.57.1206. [DOI] [PubMed] [Google Scholar]
- Obata S., Furukubo S., Kumagai I., Takahashi H., Miura K. High-level expression in Streptomyces lividans 66 of a gene encoding Streptomyces subtilisin inhibitor from Streptomyces albogriseolus S-3253. J Biochem. 1989 Mar;105(3):372–376. doi: 10.1093/oxfordjournals.jbchem.a122671. [DOI] [PubMed] [Google Scholar]
- Obata S., Taguchi S., Kumagai I., Miura K. Molecular cloning and nucleotide sequence determination of gene encoding Streptomyces subtilisin inhibitor (SSI). J Biochem. 1989 Mar;105(3):367–371. doi: 10.1093/oxfordjournals.jbchem.a122670. [DOI] [PubMed] [Google Scholar]
- Taguchi S., Kumagai I., Miura K. Comparison of secretory expression in Escherichia coli and Streptomyces of Streptomyces subtilisin inhibitor (SSI) gene. Biochim Biophys Acta. 1990 Jul 30;1049(3):278–285. doi: 10.1016/0167-4781(90)90098-m. [DOI] [PubMed] [Google Scholar]
- Taguchi S., Maeno M., Momose H. Extracellular production system of heterologous peptide driven by a secretory protease inhibitor of Streptomyces. Appl Microbiol Biotechnol. 1992 Mar;36(6):749–753. doi: 10.1007/BF00172187. [DOI] [PubMed] [Google Scholar]
- Taguchi S., Yoshida Y., Matsumoto K., Momose H. Improved leader and putative terminator sequences for high-level production of Streptomyces subtilisin inhibitor in Escherichia coli. Appl Microbiol Biotechnol. 1993 Aug;39(6):732–737. doi: 10.1007/BF00164458. [DOI] [PubMed] [Google Scholar]
- Tange T., Taguchi S., Kojima S., Miura K., Momose H. Improvement of a useful enzyme (subtilisin BPN') by an experimental evolution system. Appl Microbiol Biotechnol. 1994 Apr;41(2):239–244. doi: 10.1007/BF00186966. [DOI] [PubMed] [Google Scholar]
- Wicker C., Reichhart J. M., Hoffmann D., Hultmark D., Samakovlis C., Hoffmann J. A. Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J Biol Chem. 1990 Dec 25;265(36):22493–22498. [PubMed] [Google Scholar]





