Abstract
L-amino acid oxidase (LAAO) is a dimeric glycosylated flavoenzyme, a major constituent of the snake-venom from Calloselasma rhodostoma. The enzyme exhibits apoptosis-inducing effects as well as antibacterial and anti-HIV activities. The structure of LAAO with its substrate (L-phenylalanine) has been refined to a resolution of 1.8 Å. The complex structure reveals the substrate bound to the reduced flavin (FADred). Alternate conformations for the key residues: His223 and Arg322 are evident, suggesting a dynamic active site. Furthermore conformational changes are also apparent for the isoalloxazine ring; the three ring system exhibits more bending around the N5—N10 axis compared to the oxidized flavin. The implications of the observed dynamics on the mechanism of catalysis are discussed. Inspection of buried surfaces in the enzyme reveals a Y shaped channel system extending from the external surface of the protein to the active site. One portion of this channel may serve as the entry path for O2 during the oxidative half reaction. The second region, separated from the proposed O2 channel by the N-terminus (residues 8 – 16) of the protein, may play a role in H2O2 release. Interestingly, the later portion of the channel would direct the H2O2 product to the exterior surface of the protein, near to the glycan moiety, thought to anchor the enzyme to the host cell. This channel location may explain the ability of the enzyme to localize H2O2 to the targeted cell inducing the apoptotic effect.
Keywords: flavoenzyme, L-amino aicd oxidase, oxygen tunnel, reduced enzyme
Introduction
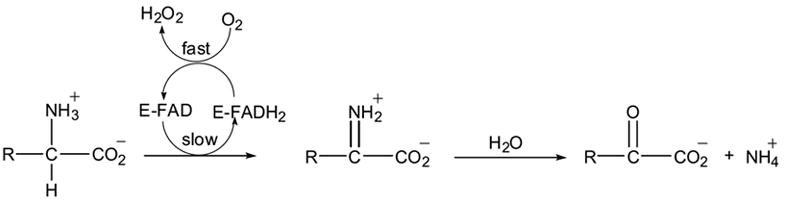
L-amino acid oxidases (LAAOs, EC 1.4.3.2) are enantioselective flavoenzymes catalyzing the oxidative deamination of a wide range of L-amino acids. During the reductive half reaction, the amino acid substrate is oxidized to the imino acid with concomitant reduction of the flavin cofactor (flavin adenine dinucleotide, FAD). The imino acid product of oxidation undergoes a non-enzymatic hydrolysis to give the respective α-keto acid and ammonia. An oxidative half reaction completes the catalytic cycle reoxidizing the FAD with molecular oxygen and producing hydrogen peroxide (Scheme 1).
Scheme 1.
L-amino acid oxidase activity has been detected in a wide range of organisms, both eukaryotic and prokaryotic.1 The LAAOs isolated from snake venoms are the most well characterized members of this enzyme family. The enzyme is found in high concentrations in venomous snakes and is thought to contribute to the toxicity of the venom. Although its exact role in toxicity is not fully understood it may be partly due to impairment of platelet aggregation, which enhances the observed injuries in snake bite victims.2 Other biological activities of LAAOs from snakes have also been reported, including apoptosis-inducing activity on various human cell lines2 as well as antibacterial3 and anti-HIV activity.4 It has been shown that these effects could be correlated to the production of a highly localized concentration of H2O2.5-7 An important feature of venomous LAAOs, as first shown for LAAO from Crotalus adamanteus, is the presence of glycosylation sites on the surface of these enzymes. Indeed, previous studies on LAAO from Crotalus atrox, expressed in eukaryotic cells, revealed that inhibition of N-glycosylation blocks its secretion to the medium and abolishes activity of the residually secreted enzyme.8 The glycan moiety of LAAO has been implicated in docking the enzyme to the surface of the host cell and enhancing the localization of high concentrations of H2O2. In fact, the finding that LAAO-induced apoptosis is distinct from that caused by exogenous hydrogen peroxide supports the idea that the mode of hydrogen peroxide delivery is an important factor in inducing apoptotic activity. Such findings have generated an increasing interest in the application of venom LAAOs as a target for the treatment of AIDS.4
LAAO has been the subject of a number of biophysical, biochemical, spectroscopic and kinetic studies (see review by Curti et al.1). Most of these studies have focused on the enzyme isolated from C. adamanteus, which shares 85% sequence identity with LAAO from C. rhodostoma (the enzyme source used in the current paper). Initially, the catalytic mechanism was proposed to be distinct from that of D-amino acid oxidase (DAAO), a functionally related enzyme that carries out the same redox reaction but with specificity to D- rather than L-amino acids as substrates.9 Subsequent studies by Massey and Curti10 suggested that the two enzymes proceed via a similar mechanism. As shown in Scheme 1, during the reductive-half reaction, the alpha hydrogen atom is abstracted by the flavin producing FADred and the imino acid. Two alternative mechanisms have been proposed: 1) a carbanion mechanism in which the proton is transferred leaving a negative charge on the alpha carbon atom, followed by the transfer of the two electrons in either one or two steps; 2) a hydride-transfer mechanism in which the hydrogen is transferred simultaneously with two electrons. Recent data from structural, mechanistic and theoretical studies, mainly performed on DAAO, are most consistent with a direct hydride transfer mechanism (for a review see Fitzpatrick and references therein11).
The crystal structure of LAAO from C. rhodostoma and from Agkistrodon halys pallas have been determined revealing a mirror symmetrical relationship between the LAAO substrate binding site and that of DAAO.12,13 This relationship supports the notion that the two enzymes undergo similar catalytic mechanisms despite their distinct stereospecificity. Slight differences in the environment around the cofactor between the two enzymes might however affect details of the redox reaction. Comparisons of the high resolution structures of DAAO in both the oxidized and reduced forms reveal no significant differences in the conformation of the isoalloxazine ring as a function of the redox state.14 Our current studies address whether structural differences are evident in LAAO as a function of the redox state of the cofactor. The ability to obtain high-resolution structures of LAAO in well-defined oxidation states is imperative to correlate structural changes with the enzyme redox state. Such a study will provide significant insights into the catalytic mechanism.
For most oxidases, the difficulty in trapping and structurally characterizing a true intermediate during the course of the oxidation reaction has resulted in a paucity of mechanistic information for the oxidative half reaction of this family of enzymes. Structural information directed at ascertaining the mechanistic parameters governing oxygen reactivity will provide important information to enhance our understanding of the general features of oxidative chemistry for this class of enzymes.
Structural studies for a number of enzymes using oxygen as a substrate have uncovered discrete routes facilitating oxygen access to the catalytic centers.15-18 Similarly, crystallographic analyses of enzymes such as catalases that utilize hydrogen peroxide as the substrate have revealed specific paths for peroxide access to the active site.19 Structural studies of amino acid oxidases in different states may help to establish whether these enzymes employ a common strategy for dealing with O2 or H2O2.
Here we report the crystal structure of LAAO from C. rhodostoma in complex with the substrate L-phenylalanine (L-Phe) refined to 1.8 Å. The structure reveals possible routes specific for O2 entry and H2O2 release to and from the active site respectively during the oxidative half reaction. These studies give insights into the features of the protein that play a role in oxidative catalysis.
RESULTS
The Phenylalanine Complex
The structure of the LAAO-Phe colorless crystals, assumed to contain the flavin cofactor in the reduced state was solved and refined to 1.8 Å resolution. The 4-fold averaged electron density difference maps, shown in Figure 1a, as well as the maps calculated by incorporating NCS-restraints into the refinement of the LAAO-Phe complex structure clearly demonstrate the presence of a bound ligand at the enzyme active site.
Figure 1.

(a) Stereo view of the sigma-A weighted 2Fo – Fc difference electron density map, averaged over four protomers in the asymmetric unit. The map is contoured around L-Phe, His223 and Arg322 at 1.2σ level and, around the isoalloxazine ring of FAD at 5.0σ level. The protein and cofactor atoms are displayed in grey bonds and the ligand is shown in tan coloured bonds. (b) Superposition of the isoalloxazine ring of the reduced (black carbon atoms) and oxidized (green carbon atoms) flavin showing the geometrical changes. A drawing of the chemical structure of the three-ring system of the flavin moiety, with the labels included for O4 and N3 is also shown.
Interpretation of the electron density maps required consideration of the nature of the bound ligand and of the oxidation state of the flavin cofactor. For the later, the colorless state of the LAAO-Phe crystals throughout the data collection implies that the flavin is predominantly in the reduced state. Generally, the flavin ring adopts a bent conformation similar to that seen in many flavoenzymes including the previously reported structure of LAAO.12 However, refining the structure of LAAO-Phe including FAD coordinates extracted from the 1F8R model, with the dihedral and planarity restraints removed from the calculations (as described in the Methods & Materials), results in changes in the isoalloxazine ring system compared to that observed in the structures of LAAO with the inhibitors, anthranilate and citrate.12 Specifically, the three-ring system is more bent around the N5—N10 axis in the LAAO-Phe structure than observed in the other complex structures. The N5 atom lies out of the plane of the pyrimidine ring, shifted by 0.13Å with respect to the position in the oxidized structures. It is directed towards the Cα of the bound ligand and adopts significant tetrahedral character, (Figure 1b). A comparison of the angle between the two planes of the isoalloxazine moiety (plane 1 defined by atoms: N5-N10-C4a-C10 and plane 2 defined by atoms: N5-N10-C5a-C9a) for the oxidized and reduced structures reveals significant differences; 171.24° ± 0.74° and 162.55° ± 1.49° for for FADox and FADred respectively. Thus the reduced form of the cofactor is more bent than the oxidized form. These structural changes to the isoalloxazine ring may be indicative of the reduced flavin in LAAO.
Inspection of the ligand density was undertaken to determine if it corresponds to the amino or imino form. The alpha carbon atom of the imino form is expected to adopt a planar conformation (N, Cα, Cβ and C lying in the same plane) since it is sp2 hybridized. In contrast, the amino form of L-phenylalanine would adopt a tetrahedral geometry at the alpha carbon atom due to sp3 hybridization at the alpha carbon atom. In order to clearly distinguish between these two geometric states at the alpha carbon atom, the angular, dihedral and chirality restraints were removed from the ligand in the final round of refinement (see Materials and Methods). As shown in Figure 2, the alpha carbon atom adopts a tetrahedral geometry, however the angles around this carbon atom do not correspond to those of an ideal tetrahedron (∠NCαC = 115.6°, ∠NCαCβ = 97.2°, ∠CβCαC = 134.2°). Computation of the chiral volume for the alpha-carbon of L-Phe, revealed an average value of 1.9 ± 0.1 for the four bound ligands (as compared to a chiral volume of 2.5 for an ideal tetrahedral configuration). Favorable interactions with active site residues stabilize this distorted geometry of the bound ligand. Enforcing the imino form during refinement resulted in a decreased quality of density for the ligand. Therefore, based on these observations, we conclude that the structure of the LAAO-Phe corresponds, predominantly to the reduced flavin in complex with the substrate. This observation supports previous kinetic studies, which suggest that under conditions of high substrate concentration, the ligand binds to the reduced enzyme to form a non-productive complex resulting in inhibition of the enzyme.10
Figure 2.
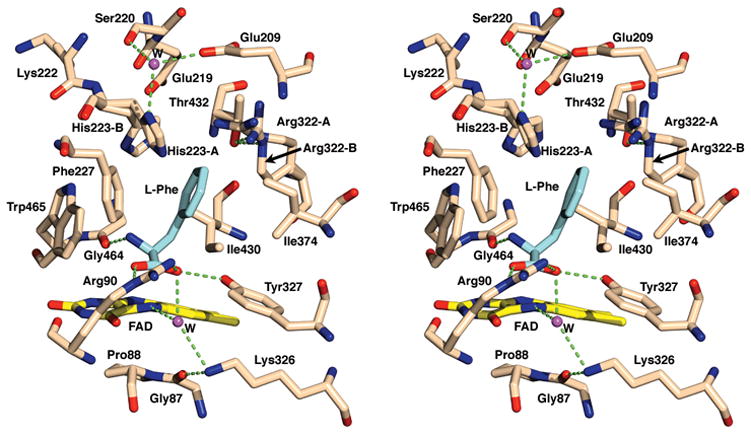
Stereo view of the catalytic site showing the interactions between LAAO and the L-phenylalanine ligand. The alternate conformations of His223 and Arg322 are designated as A and B. Hydrogen bonds are shown as green dashed lines. The substrate is shown in grey bonds and the protein and FAD are shown in tan colored bonds. Water molecules have been included as magenta spheres and labeled W.
Dynamic active site
Alternate conformations of two active site residues (His223 and Arg322) are evident in the difference electron density maps providing insights into the dynamics of the enzyme and suggesting possible movements relevant to the catalytic process (Figure 1a). Both of these residues are located along the entry pathway for the substrate into the active site. The most significant movement is observed for His223; the side chain moves by ~90o within the binding cavity (conformations A and B in Figure 2). The refined occupancies are approximately 40% and 60% for conformations A and B respectively. In conformation A the imidazole side chain is held in position by water-mediated hydrogen bonding interactions involving Glu209 and Ser220. The imidazole ring of conformation B of His223 lies closer to the phenyl ring of the ligand. The plane of the imidazole ring and that of the phenyl ring are at a slight angle from each other, with the closest point of contact at 3.1 Å. Although not a complete π-stacking interaction, these contacts may aid in correctly orienting the substrate at the catalytic center. The lack of direct hydrogen bond interactions with either the main chain or discrete side chain atoms may increase the mobility of this residue. The movements of the imidazole side chain may be correlated with the proposed role of His223 as the base to deprotonate the α-NH3+ group of the amino acid substrate12 as discussed below.
A less pronounced conformational change is observed for Arg322; the two observed conformations (A and B in Figure 2) differ with respect to the positions of Cγ and Cδ as reflected in their χ1 angles (χ1 = −84° vs. 73° for A and B, respectively). In contrast, the position of the terminal guanidinium portion of the side chain is similar in both conformations, with the Nε atom, held in position by a hydrogen bond interaction with the hydroxyl oxygen atom of Thr432. This Thr432-Nε interaction anchors the terminal guanidinium nitrogen atoms of both conformations, in approximately the same location. The guanidinium group may act to stabilize the negative charges from the nearby side chains of Glu209 and Glu219. Furthermore, changing the conformation of Arg322 from A to B results in favorable van der Waals interactions between the aliphatic portion of the arginine side chain and that of the L-phenylalanine ligand. Thus the observed movements of the side chains of His223 and Arg322 may be related to the binding and release of substrate and product respectively (see below for further discussion).
Interactions between L-phenylalanine and Reduced-LAAO in the Active Site
The interactions of L-phenylalanine (Km = 50 μM, at pH 8.5 and 25°)20 at the active site are depicted in Figure 2. The ligand is bound at the re-face of the cofactor with the side chain extending away from the cofactor and the carboxylate group engaged in a salt-bridge interaction with the guanidinium group of Arg90, and a hydrogen bond with the hydroxyl group of Tyr372. This binding mode is similar to that observed in the structures of DAAO14 and flavocytochrome b2 (FCb2).21 In addition, one of the two oxygen atoms of the substrate carboxylate group is involved in a water-mediated interaction with the flavin-N5 as well as the side chain amino group of Lys326. The alpha carbon atom of the substrate, representing the site of oxidative attack, is positioned over the pyrimidine portion of the isoalloxazine ring at a distance of 3.1 Å from N5. The amino group of the substrate forms a hydrogen bond interaction with the carbonyl oxygen atom of Gly464. Finally, the side chain of the ligand participates in hydrophobic interactions with the side chains of Ile430, Ile374, Phe227, the aliphatic portion of the side chain of Arg322 in conformation B and with the imidazole ring of His223 in conformation B, as discussed above.
Two important structural points can be highlighted from these detailed interactions. First, the distance between the oxidative center of the ligand and flavin-N5 falls within the same range observed for other flavoenzymes (average 3.5 Å), a recurrent feature of these enzymes.22 Such a close distance is relevant to the proposed direct hydride transfer mechanism as will be discussed in more detail below. Second, the triad (Lys326/water/flavin-N5) observed in LAAO is similar to that found in other flavoenzymes including polyamine oxidase,23 monoamine oxidase B,24 and monomeric sarcosine oxidase.25,26 This feature, however, is not completely conserved amongst all flavoenzymes.24 Whether this conserved structural water plays a mechanistic role in the redox reaction remains unclear.
Structural analysis of protein-ligand interactions at the catalytic site forms a strong basis for understanding the underlying mechanism of the enzymatic reaction and it provides an explanation for the observed specificity of LAAO. Studies on LAAO report that aromatic or more generally hydrophobic amino acids are preferred; deamination of polar and basic amino acids proceeds at a much lower rate (see Curti and references therein1). This suggests that introducing a positively charged amino acid substrate such as lysine or arginine would result in unfavorable electrostatic interactions with Arg322. Clearly, hydrophobic amino acids suit perfectly the hydrophobic binding pocket in the catalytic cavity. The presence of bulky substituents at the phenylalanine ring have been shown to slow the enzymatic reaction presumably due to steric clashes at the active site that affect the correct substrate orientation.27
Possible Inlet and Outlet for Oxygen and Hydrogen Peroxide
The oxidative half reaction of LAAO utilizes O2 to regenerate the oxidized cofactor and produces H2O2 (see Scheme 1). During a single catalytic cycle, the enzyme must process two substrates (an amino acid and O2). The order of events involves reaction of the amino acid first (reductive half reaction), followed by reaction with O2 (oxidative half reaction). Whether the oxygen binds after the release of the imino product or a ternary complex of (imino acid-reduced enzyme-O2) is involved in the catalytic mechanism is unclear; a survey of the literature shows contradicting results.10,28 Given this sequence of events during the catalytic cycle, the manner in which oxygen accesses the catalytic cavity of the enzyme raises an important issue. The structure clearly reveals that binding of the amino acid substrate results in a sealed catalytic cavity with no obvious access for oxygen through the amino acid entry channel to the flavin moiety. Our structural studies are directed at establishing how the enzyme allows oxygen access to the catalytic site.
Inspection of the buried accessible surface of the crystal structure of LAAO (Figure 3), calculated by GRASP,29 reveals a water filled channel (with the narrowest region exhibiting a radius of 1.5 Å), different from that used for the substrate entry; this channel links the substrate cavity to the external environment. The same channel was predicted by the programs VOIDOO30 and CAVER.31 The channel is located between the FAD-binding and helical domains, starting from a position near to the substrate binding site, passing underneath the □9-helix of the helical domain. The exit of the channel to the bulk solvents is located within the substrate-binding domain near to the N-terminus (channel A in Figure 4). This water filled channel, bounded predominately by hydrophobic residues and the non-polar portions of polar side chains has significant hydrophobic character (Figures 3 and 4b). Sequence alignment of LAAO from C. rhodostoma with LAAOs from other sources reveals conservation of two channel residues: His95 and Arg96 (data not shown). Hydrophobic residues equivalent to Tyr16 and Ile226 lining the channel are also present in other LAAOs. The hydrophobic nature of the channel suggests that it may form the path for O2 to the catalytic site. Indeed, hydrophobic channels that function as access routes for oxygen molecules have been observed in two flavoenzyme oxidases15,16 as well as other oxygen dependent enzymes.17,18 Interestingly, the accessibility of oxygen to the catalytic site through this channel appears to be regulated by the side chain movement of His223, conformation A of which blocks its access.
Figure 3.

Stereo representation of the accessible surface (AS) calculated in GRASP.35 Hydrophobic and polar regions of the surface are colored in yellow and red respectively. The routes labeled S and O trace the pathways of the amino acid substrate through the main entry channel and O2 through the proposed oxygen channel respectively. Continuity between the active site and the oxygen channel is broken by the side chains of Ala462 and Ile226 and the main oxygen atom of Lys222. His223 has been modeled in conformation B, obstructing the amino acid entry channel.
Figure 4.
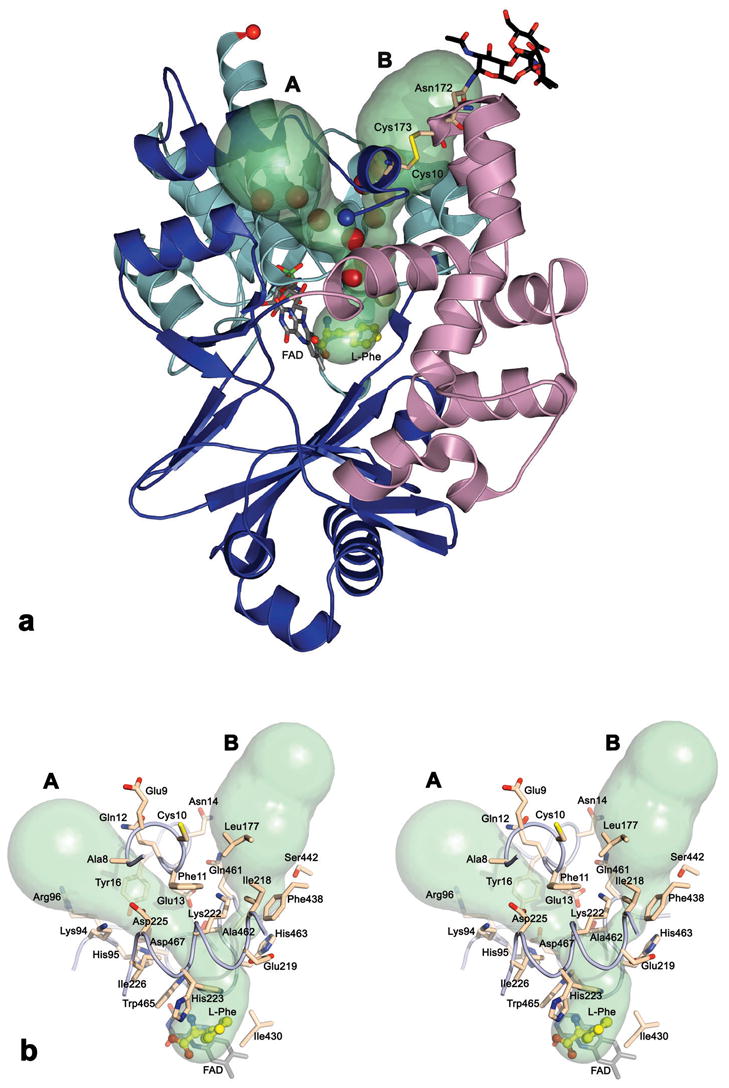
(a) Ribbon and surface representation depicting the proposed O2 entry channel (A) and the H2O2 exit channel (B) in the LAAO structure. The structural elements making up the FAD-binding domain are colored in cyan, the substrate-binding domain is colored blue and the helical domain is in magenta. The channels are displayed as a molecular surface calculated by CAVER31, and filled with water molecules (red spheres). The bound ligand is displayed as a ball-and-stick model with carbon atoms colored yellow; the cofactor, disulfide bridge between Cys10 and Cys173 and the glycan moiety bonded to Asn172 are shown as a stick representation. The N and C termini of LAAO are indicated by blue and red spheres, respectively. (b) A stereo view showing the residues lining the proposed O2 and H2O2 channels. Both side chain conformations have been included for His223 although the surface has been calculated with His223 in conformation B. The coloring of the atoms is as described for figure (a). Slight changes in the view orientations of the (a) and (b) figures have been made to maximize clarity.
Additionally, visual investigation of the crystal structure reveals a large surface cavity, filled with water molecules, separated from the channel by the N-terminal residues 8 – 16 (Figure 4b). This region of the structure is restricted from movement by a disulfide bridge between Cys10 and Cys173. The water molecules filling this cavity have no direct access to the active site, however, the calculated molecular surface indicates a linkage, between the proposed oxygen channel and the surface cavity, forming a bifurcated channel as shown in Figure 4. Moreover, using a probe of 1.4 Å radius, the program CAVER predicts a pathway (channel B in Figure 4) from a point near the substrate location towards the outside suggesting a second putative channel. These two channels (A and B) form a Y-shaped system. The common stem of this Y shaped channel is approximately 9Å in length and extends from the substrate-binding cavity to the bifurcation point. This second channel passes below the □9-helix along the side chain of Lys222. We suggest that the second pathway (channel B) might function as an exit for the H2O2 product during the oxidative half reaction. The existence of specific paths for H2O2 has previously been shown in catalases, where peroxide acts as a substrate.20 Comparison of the residues that line the catalase peroxide channel and the observed channel in LAAO reveal predominantly hydrophobic residues or the aliphatic portions of polar residues, as expected to suit the less polar H2O2 molecule (dipole moment = 1.57 D). Interestingly, the path suggested for peroxide release in LAAO is positioned near to the glycosylation site at Asn172. This may suggest a rational for the apoptotic inducing activity observed in LAAO.5-8 Such a structural arrangement would result in a high concentration of released H2O2 at the targeted cell surface thus enhancing the induced apopotic effect. The existence of such an exit would require the residues forming the channel to be displaced in order to allow the passage of a hydrogen peroxide molecule. Preliminary TLS analysis shows that the helical domain exhibits a rigid body movement relative to the other two domains constituting the LAAO structure, as is evident by comparing the eigenvalues of the liberation tensor (L) for the three domains. For monomer A this analysis gives the following L-eigenvalues: L = 3.781, 1.039, 0.545 deg2 for the helical domain, L = 1.448, 0.908, 0.531 deg2 for the substrate domain and L = 1.179, 1.559, 1.328 deg2 for the FAD domain. Similar behavior has been observed for the other monomers in the asymmetric unit.
Discussion
The structure of LAAO bound to the L-phenylalanine substrate highlights important features of this flavoenzyme and provides a deeper understanding of the catalytic mechanism. The identification of the chemical nature of the bound ligand as well as the oxidation state of the flavin cofactor is crucial to the analysis and structural interpretation. The observation that the crystals bleached upon exposure to L-phenylalanine, and remained colorless during the data collection process, coupled with the geometrical features obtained from refinement of both the bound ligand and the FAD, strongly support the interpretation that the substrate is bound to the reduced enzyme.
In the presence of L-phenylalanine, the enzyme is capable of turnover within the crystal state. At room temperature, one might expect the bleached crystals (reduced cofactor) to become reoxidized in the presence of dissolved oxygen in the crystal stabilizing solution. However, the concentration of L-phenylalanine used in the crystal soak solution is 150 times molar excess to that of dissolved oxygen in solution (0.3mM). This large excess of phenylalanine might result in binding of substrate prior to reoxidation of the cofactor. This interpretation supports previous suggestions that the observed inhibition of LAAO at high substrate concentration could be due to binding of the substrate to the reduced flavin.10
Comparisons of the oxidized and reduced forms of the cofactor for different flavoenzymes do not indicate a correlation between the redox state and the cofactor geometry38 however the protein microenvironment does play a critical role in the geometric features of the cofactor. Indeed, it is likely that this constitutes a role in fine-tuning the redox potential for the cofactor. Structures of glycolate oxidase,32 and p-hydroxybenzoate hydroxylase,33 have revealed large movements of the isoalloxazine ring, exposing it to the solvent during the oxidative-half reaction. In contrast, the very high resolution structures of DAAO revealed no detectable differences between the oxidized and reduced forms of the cofactor.14 In our structure of the LAAO/L-phenylalanine complex the change in the cofactor geometry correlated with the reduced form of the enzyme suggest that cofactor geometry does play an important role in redox chemistry in certain cases. Indeed, recent atomic resolution structural studies on cholesterol oxidase in the presence of a substrate analogue also reveal geometric changes to the cofactor induced by the binding of the ligand (Lyubimov & Vrielink, personal communication). Furthermore, theoretical studies by Hall and coworkers have shown that the redox state of the cofactor does affect geometry.34
The pattern of substrate interactions in LAAO is nearly identical to that observed in DAAO (pdb code 1C0L). As discussed earlier by Pawelek et al.,12 the active sites in these two amino acid oxidases possess a high degree of enantiomeric conservation, relevant to the stereospecificity requirements of the substrate; Arg287, Tyr240 and Ser336 in the mirror image structure of DAAO correspond to Arg90, Tyr372 and Gly464, respectively, in LAAO. Intriguingly, the mode of ligand binding in amino acid oxidases is similar to the related FMN-dependent flavoenzyme: flavocytochrome b2 (FCb2) (pdb code 1SZF). FCb2 belongs to the α-hydroxyacid oxidase family, which oxidizes the CO rather than the C—N bond and binds to their ligands from the si-face of the isoalloxazine ring.35 Despite apparent differences, the carboxylate groups of the ligands form salt-bridge interactions with arginine residues (Arg376 in 1SZF corresponding to Arg90 in LAAO). Furthermore, the substrate amino nitrogen atom in amino acid oxidases and the substrate oxygen atom in FCb2 are held in position via hydrogen bond interactions. The hydroxyl oxygen atom to be deprotonated by His373 in FCb2 acts as a hydrogen bond acceptor interacting with Tyr254-OH in 1SFZ36; whereas the amino N-atom in amino acid oxidases functions as a hydrogen bond donor to a backbone oxygen atom (Gly464-O in LAAO and Ser336-O in DAAO). Finally, in all three flavoenzymes compared, the hydrophobic portion of the substrate lies in a hydrophobic pocket within the enzyme active site. These structural similarities at the active sites of flavin-dependent oxidases suggest a common mechanism for oxidation chemistry as proposed by recent studies.11
The crystal structure of LAAO reveals significant conformational changes for His223 and Arg322, emphasizing the dynamic nature of the enzyme. These movements may be correlated to the binding and release of the ligand. Initial substrate binding may induce His223 to adopt conformation A (O2 access to the active site is blocked), to allow substrate entry and deprotonation of the zwitterion. When the substrate is in the Michaelis position, conformation B (oxygen access channel is open) would be preferred due to favorable interactions between the imidazole and phenyl moieties. However this preference is not absolute since, while the occupancy of L-Phe refines to ≥85%, His223 is still found in a mixed state (40%/60% in conformation A/B respectively). The significant proportion of conformation A may be a result of the large excess of the substrate in the crystal soaking solution and may explain why the crystals remain in the reduced colorless state for an extended period of time; reoxidation, evidenced by a return of the crystals to the yellow color, takes several hours.
The high pKa for the α-amino group (9.0 – 10.5) suggests that the substrate binds to the enzyme as a zwitterion under physiological conditions. Indeed a study on the effect of pH on the reduction of LAAO by L-phenylalanine is consistent with this hypothesis, suggesting that binding of the zwitterion results in protonation of a base in the enzyme.28 Furthermore, kinetic studies of LAAO from C. adamanteus, investigating the effect of pH on Km, suggest an ionizable group in the enzyme-substrate complex, with a pKa of 5.7 – 5.9, assigned to a histidine residue at the catalytic site.27 The position of His223 in the active site of the enzyme suggests it may play the role of the proposed catalytic base. The protonation state of His223 may induce a movement of the side chain between conformation A (oxygen access from the channel to the active site is blocked) and B (amino acid channel is blocked). Given that the structure represents a time averaged view of the enzyme, and that the structure may also represent a mixture of ligand states, the full details of the dynamic behavior of this side chain cannot be obtained by a crystallographic study.
Two conformations are observed for Arg322. Movement of the side chain from conformation A to B (Figure 2) produces favorable hydrophobic interactions between the aliphatic portion of the side chain and the aromatic ring of L-phenylalanine. Reversing the position of Arg322 from B to A would weaken the interaction with the product thus facilitating its release.
Careful analysis of the refined structure reveals a channel that may function in oxygen access; in addition, a model for a possible pathway to release H2O2 is suggested (channel B in Figure 4). The hydrophobic nature of the proposed oxygen channel and its location with respect to the cofactor is consistent with this functional role. It should be noted that the small radius of this channel rules out the possibility that it forms an exit route for the imino product. The LAAO-Phe complex structure shows that there is no space for O2 to enter the active site through the main channel and access the reduced cofactor to complete the oxidative half reaction. Indeed, release of the imino product from the catalytic cavity is required if O2 were to access the cofactor through the main channel. In contrast, the proposed oxygen channel provides an alternative for O2 entry without requiring prior product release.
The alternative conformations of His223 may function by gating entry of the amino acid and O2 substrates to the active site cavity. The imidazole ring of His223 in conformation A blocks the O2 channel and widens the amino acid entry channel to facilitate the passage of the substrate. In contrast, conformation B of His223 obstructs the amino acid entrance and opens the O2 entry and H2O2 release route. The position of the imidazole ring may therefore be controlled by the presence of the bound ligand. Such alternate conformations have already been observed in the anthranilate complex structure of LAAO whereas, in the citrate complex structure, the His223 is locked in conformation B due to H-bonding interaction with the bound citrate molecule.12
In addition to the proposed O2 channel, a model for H2O2 exit is suggested. The relative position of the glycan moiety at Asn172, with respect to the proposed H2O2 channel provides rational for the powerful inducing-apoptotic effect of LAAO on various cell lines. As mentioned above, the glycosylation of LAAOs has been shown to be critical for the apoptotic activity.8 Presumably, anchoring the enzyme, via its glycan moiety, to the host cells combined with the localized release of H2O2 affords an efficient mechanism to destroy the cell. This proposed model remains to be further tested.
Structural Insight into Mechanism
The crystallographic structure of LAAO bound to the L-phenylalanine provides comprehensive insights into the catalytic redox reaction of LAAO (Figure 5). The protonated amino acid enters the catalytic site through the main funnel shaped channel as previously proposed by Pawelek et al.12 however, the substrate is blocked midway along this channel by a constriction composed of the side chains of His223 and Arg322. Induced by the presence of the zwitterionic form of the substrate, His223 and Arg322 alter their side chain conformations. The α-amino group of the substrate is deprotonated by His223. The distance of the imidazole moiety of His223, in either conformation, from the amino group of the substrate in the bound position in the complex structure suggests that deprotonation might occur prior to substrate binding at the Michaelis position. Once deprotonated, the substrate is activated for further chemistry; movement of the lone pair of electrons from the amino nitrogen atom to the alpha carbon atom to form the imine.
Figure 5.
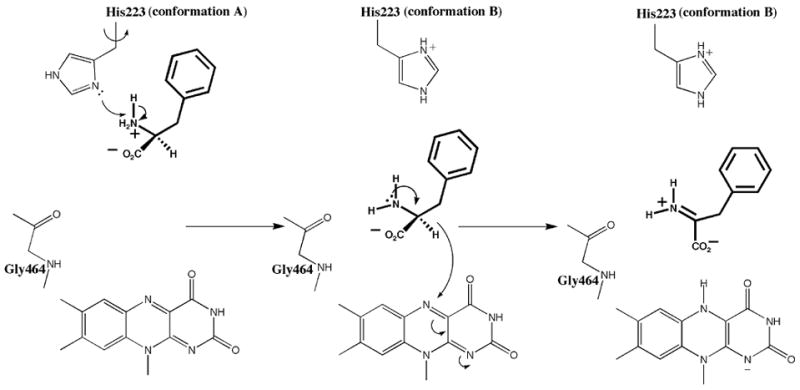
Reaction mechanism for the oxidation of L-phenylalanine by L-amino acid oxidase. The substrate is shown in thick bonds. Two conformations for His223 are included as labeled.
The Michaelis complex of the enzyme may consist of the oxidized enzyme with the amino acid substrate bound in a distorted tetrahedral geometry around the substrate alpha carbon atom. The distorted geometry is stabilized by the favorable interactions at the active site. The distortion of the bound substrate from fully tetrahedral to partially planar, with the alpha carbon atom adopting some sp2 character, facilitates the oxidation of the amino acid by approaching a geometry that more closely mimics that of the imino product. In addition, distortion of the isoalloxazine ring of the cofactor results in an ideal arrangement for direct hydride transfer from the substrate alpha carbon to N5 of the flavin moiety, to yield the imino acid and FADred. Thus the Michaelis complex represents a geometry that more closely mimics the transition state for substrate oxidation.
During the oxidative half reaction, it is uncertain whether oxygen binds after release of the imino product or if a ternary complex (imino acid-reduced enzyme-O2) is involved in the catalytic mechanism,1,10 Nevertheless, the presence of the proposed O2 channel can be supported by either mechanism. The enzyme may employ the channel for oxygen binding either before or during product release.
The crystallographic structure reveals a conserved water molecule, part of the triad lys326/water-/enzyme, observed in many flavoenzymes, which acts as a hydrogen bond donor to N5 of FAD and one of the oxygen atoms of the substrate carboxylate group, and accepts a hydrogen atom from the side chain amino group of Lys326. These interactions are disrupted upon the completion of the reductive half reaction, since N5 acquires a hydride from the substrate and the imino product is ready to exit the catalytic site. In a recent kinetic study on Maize POA, this conserved water has been proposed to play a role in the reductive half reaction.37 Alternatively, this water may assist in H2O2 formation from the flavin-hydroperoxy intermediate, assumed to be formed during the oxidation of FADred. Chemical studies have shown that protic solvents facilitate the hydrolysis of the flavin-hydroperoxy intermediate.38 Clearly, more work directed at studying the oxidative half reaction of flavoenzymes are necessary to delineate a detailed picture of the mechanism.
In summary, the structure of the reduced form of LAAO in the presence of the phenylalanine substrate has provided insights into the mode of substrate binding as well as the mechanism of enzymatic activity. Conformational changes of the key active site residues, His223, Arg322 and the FAD cofactor, have been observed and can be related to the reaction mechanism. Additionally, structural analysis has uncovered a channel, proposed as an entry access for the O2 substrate during the reductive half reaction. Finally a structural model has been presented for H2O2 release near to the glycan moiety at Asn172. This may account for the apoptotic-induced activity observed for LAAO.
MATERIALS AND METHODS
Protein purification and crystallization
LAAO from C.rhodostama was purified from isolated snake venom as outlined by Ponnudari et al.20 Prior to crystallization, the enzyme, stored at −80 oC, was thawed and subjected to a reactivation process by incubating at 30 oC after dialysis against 200 mM acetate buffer (pH 4.6). The enzyme activity was confirmed using a horseradish peroxidase coupled assay as described in the purification paper. The reactivated enzyme was dialyzed exhaustively against a buffer containing 25 mM MOPS pH 7.0 and 100 mM NaCl, and concentrated to 5 mg/ml as determined by Bradford assay using bovine serum albumin as a standard.
Crystals of the free enzyme were obtained within a few days using the hanging-drop vapor diffusion method at 17 oC from 20 – 22% polyethylene glycol (PEG) 4000, 200 mM Li2SO4, 10% glycerol and 100 mM Tris-HCl pH 7.4. The complex of LAAO with the substrate (L-phenylalanine) was obtained by soaking crystals of the native enzyme for 4 hours in the crystallization solution containing 50 mM L-phenylalanine. During this soaking procedure the yellow, oxidized, enzyme crystals bleached to give colorless crystals, suggestive of a reduced enzyme complex. These crystals remained colorless throughout the entire data collection.
X-ray diffraction, Data collection and Processing
Crystals used for data collection were flash-frozen by transferring directly from the mother liquor into the cryo-stream of nitrogen gas at 83 K. Diffraction data for the LAAO-Phe complex crystals were collected at beamline 19-ID, the Advanced Photon Source, Argonne National Laboratory. All data were integrated and scaled using the HKL suite of software.39 Subsequent calculations were performed with programs from the CCP4 suite.40 The data collection and processing statistics are given in Table 1.
Table 1.
Data collection and refinement statistics
| Data set | LAAO-Phe complex |
|---|---|
| Cell (Å) | |
| a | 78.8 |
| b | 154.0 |
| c | 103.2 |
| β(°) | 109.5 |
| Space group | P21 |
| Resolution (Å) | 50 – 1.8 |
| Number of observations | 3,664,879 |
| Number of unique reflections | 211,566 |
| Completeness (top shell) (%) | 99.5(100) |
| Rmergea (top shell) (%) | 10.2(36.4) |
| R-factorb | 0.16 |
| Rfreec | 0.20 |
| No. of non-hydrogen atoms | |
| protein | 15,436 |
| FAD | 212 |
| L-phenylalanine | 48 |
| carbohydrate | 142 |
| water | 2085 |
| Average B-factors (Å2) | |
| protein atoms | 18.6 |
| FAD atoms | 10.9 |
| L-phenylalanine atoms | 29.6 |
| carbohydrate atoms | 44.6 |
| water molecules | 23.2 |
| Model quality: | |
| rmsd bond lengths (Å) | 0.013 |
| rmsd bond angles (°) | 1.489 |
| Estimated coordinate error (Å) | 0.118 |
| Ramachandran analysis (%): | |
| Favored/allowed regions | 98.2/1.8 |
where the sum i is over all separate measurements of the unique reflections hkl.
R-factor = Σhkl||Fobs − |Fcalc|| / Σhkl|Fobs|.
Rfree as R-factor but summed over a 5% test set of reflections.
Model Building and Refinement
The LAAO-Phe complex was refined starting with the deposited coordinates of LAAO (pdb code 1F8R12) and removing the citrate and water molecules from the model; the FAD cofactor was included in the model at the onset of refinement. The model was refined against the crystallographic data within the resolution range 50 – 1.8 Å. Further phase improvement was obtained by alternating cycles of refinement, using the program REFMAC5,41 and manual rebuilding with O.42 TLS parameters were included in the refinement protocol with each domain (substrate, FAD-binding, and helical) treated as a rigid TLS group. TLS analysis was performed with the program TLSANL.43 Water molecules were automatically added using ARP/WARP.44 The ligands and the carbohydrate chains were incorporated after inspection of the SigmaA-weighted difference maps. Applying NCS restraints to the refinement enhanced the ligand density in the calculated maps. In the final stages of refinement, the NCS restraints were removed; the dihedral and planarity restraints were removed for the FAD cofactor by setting the target e.s.d. to unrealistically large values. Similarly, angular, torsional and chirality restraints were removed for the N, Cα, Cβ and COO portions of the substrate during the final round of refinement. Partial occupancies, chiral volumes and estimated standard deviations were determined using the program SHELX.45 The refinement statistics are given in Table 1.
Acknowledgments
We thank Sandro Ghisla for providing the enzyme for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curti B, Ronchi S, Simonetta MP, Muller F, editors. D- and L-amino acid oxidases. Vol. III. Chemistry and Biochemistry of Flavoenzymes. Boca Raton: CRC Press; 1992. [Google Scholar]
- 2.Du XY, Clemetson KJ. Snake venom L-amino acid oxidases. Toxicon. 2002;40:659–665. doi: 10.1016/s0041-0101(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 3.Stiles BG, Sexton FW, Weinstein SA. Antibacterial effects of different snake venoms: purification and characterization of antibacterial proteins from Pseudechis australis (Australian king brown or mulga snake) venom. Toxicon. 1991;29:1129–1141. doi: 10.1016/0041-0101(91)90210-i. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YJ, Wang JH, Lee WH, Wang Q, Liu H, Zheng YT, Zhang Y. Molecular characterization of Trimeresurus stejnegeri venom L-amino acid oxidase with potential anti-HIV activity. Biochem Biophys Res Commun. 2003;309:598–604. doi: 10.1016/j.bbrc.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Suhr SM, Kim DS. Identification of the snake venom substance that induces apoptosis. Biochem Biophys Res Commun. 1996;224:134–139. doi: 10.1006/bbrc.1996.0996. [DOI] [PubMed] [Google Scholar]
- 6.Suhr SM, Kim DS. Comparison of the apoptotic pathways induced by L-amino acid oxidase and hydrogen peroxide. J Biochem (Tokyo) 1999;125:305–309. doi: 10.1093/oxfordjournals.jbchem.a022287. [DOI] [PubMed] [Google Scholar]
- 7.Murakawa M, Jung SK, Iijima K, Yonehara S. Apoptosis-inducing protein, AIP, from parasite-infected fish induces apoptosis in mammalian cells by two different molecular mechanisms. Cell Death Differ. 2001;8:298–307. doi: 10.1038/sj.cdd.4400811. [DOI] [PubMed] [Google Scholar]
- 8.Torii S, Yamane K, Mashima T, Haga N, Yamamoto K, Fox JW, Naito M, Tsuruo T. Molecular cloning and functional analysis of apoxin I, a snake venom-derived apoptosis-inducing factor with L-amino acid oxidase activity. Biochemistry. 2000;39:3197–3205. doi: 10.1021/bi992416z. [DOI] [PubMed] [Google Scholar]
- 9.Wellner D, Meister A. Studies on the mechanism of action of L-amino acid oxidase. J Biol Chem. 1961;236:2357–2364. [PubMed] [Google Scholar]
- 10.Massey V, Curti B. On the reaction mechanism of Crotalus adamanteus L-amino acid oxidase. J Biol Chem. 1967;242:1259–1264. [PubMed] [Google Scholar]
- 11.Fitzpatrick PF. Carbanion versus hydride transfer mechanisms in flavoprotein-catalyzed dehydrogenations. Bioorg Chem. 2004;32:125–139. doi: 10.1016/j.bioorg.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Pawelek P, Cheah J, Coulombe R, Macheroux P, Ghisla S, Vrielink A. The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site. EMBO Journal. 2000;19:4204–4215. doi: 10.1093/emboj/19.16.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Teng M, Niu L, Wang Y, Liu Q, Huang Q, Hao Q, Dong Y, Liu P. Purification, partial characterization, crystallization and structural determination of AHP-LAAO, a novel L-amino-acid oxidase with cell apoptosis-inducing activity from Agkistrodon halys pallas venom. Acta Crystallogr D Biol Crystallogr. 2004;60:974–977. doi: 10.1107/S0907444904000046. [DOI] [PubMed] [Google Scholar]
- 14.Umhau S, Pollegioni L, Molla G, Diederichs K, Welte W, Pilone MS, Ghisla S. The x-ray structure of D-amino acid oxidase at very high resolution identifies the chemical mechanism of flavin-dependent substrate dehydrogenation. Proc Natl Acad Sci U S A. 2000;97:12463–12468. doi: 10.1073/pnas.97.23.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulombe R, Yue KQ, Ghisla S, Vrielink A. Oxygen access to the active site of cholesterol oxidase through a narrow channel is gated by an Arg-Glu pair. Journal of Biological Chemistry. 2001;276:30435–30441. doi: 10.1074/jbc.M104103200. [DOI] [PubMed] [Google Scholar]
- 16.Lario PI, Sampson N, Vrielink A. Sub-atomic resolution crystal structure of cholesterol oxidase: what atomic resolution crystallography reveals about enzyme mechanism and the role of the FAD cofactor in redox activity. J Mol Biol. 2003;326:1635–1650. doi: 10.1016/s0022-2836(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 17.Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8-Angstrom Resolution of Cytochrome C Oxidase from Paracoccus Denitrificans. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 18.Soulimane T, Buse G, Bourenkov GP, Bartunik HD, Huber R, Than ME. Structure and mechanism of the aberrant ba(3)-cytochrome c oxidase from Thermus thermophilus. Embo Journal. 2000;19:1766–1776. doi: 10.1093/emboj/19.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mate MJ, Zamocky M, Nykyri LM, Herzog C, Alzari PM, Betzel C, Koller F, Fita I. Structure of catalase-A from Saccharomyces cerevisiae. J Mol Biol. 1999;286:135–149. doi: 10.1006/jmbi.1998.2453. [DOI] [PubMed] [Google Scholar]
- 20.Ponnudurai G, Chung MCM, Tan NH. Purification and properties of the L-amino acid oxidase from Malayan Pit Viper (Calloselasma rhodostoma) venom. Archives of Biochemistry and Biophysics. 1994;313:373–378. doi: 10.1006/abbi.1994.1401. [DOI] [PubMed] [Google Scholar]
- 21.Xia ZX, Mathews FS. Molecular structure of flavocytochrome b2 at 2.4Å resolution. J Mol Biol. 1990;212:837–863. doi: 10.1016/0022-2836(90)90240-M. [DOI] [PubMed] [Google Scholar]
- 22.Fraaije MW, Mattevi A. Flavoenzymes: diverse catalysts with recurrent features. Trends Biochem Sci. 2000;25:126–132. doi: 10.1016/s0968-0004(99)01533-9. [DOI] [PubMed] [Google Scholar]
- 23.Binda C, Coda A, Angelini R, Federico R, Ascenzi P, Mattevi A. A 30-angstrom-long U-shaped catalytic tunnel in the crystal structure of polyamine oxidase. Structure Fold Des. 1999;7:265–276. doi: 10.1016/s0969-2126(99)80037-9. [DOI] [PubMed] [Google Scholar]
- 24.Binda C, Mattevi A, Edmondson DE. Structure-function relationships in flavoenzyme-dependent amine oxidations: a comparison of polyamine oxidase and monoamine oxidase. J Biol Chem. 2002;277:23973–23976. doi: 10.1074/jbc.R200005200. [DOI] [PubMed] [Google Scholar]
- 25.Trickey P, Basran J, Lian LY, Chen Z, Barton JD, Sutcliffe MJ, Scrutton NS, Mathews FS. Structural and biochemical characterization of recombinant wild type and a C30A mutant of trimethylamine dehydrogenase from methylophilus methylotrophus (sp. W(3)A(1)) Biochemistry. 2000;39:7678–7688. doi: 10.1021/bi9927181. [DOI] [PubMed] [Google Scholar]
- 26.Zhao G, Jorns MS. Ionization of zwitterionic amine substrates bound to monomeric sarcosine oxidase. Biochemistry. 2005;44:16866–16874. doi: 10.1021/bi051898d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page DS, van Etten RL. L-amino acid oxidase. III. Substrate substituent effects upon the reaction of L-amino acid oxidase with phenylalanines. Bioorganic Chemistry. 1971;1:361–373. [Google Scholar]
- 28.Porter DJT, Bright HJ. Interpretationof the pH dependence of flavin reduction in the L-amino acid oxidase reaction. J Biol Chem. 1980;255:2969–2975. [PubMed] [Google Scholar]
- 29.Nicholls A, Sharp K, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 30.Kleywegt GJ, Jones TA. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr D Biol Crystallogr. 1994;50:178–185. doi: 10.1107/S0907444993011333. [DOI] [PubMed] [Google Scholar]
- 31.Petrek M, Otyepka M, Banas P, Kosinova P, Koca J, Damborsky J. A new tool to explore the routes from protein clefts, pockets and cavaties. BMC Bioinformatics. 2006 doi: 10.1186/1471-2105-7-316. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenberg K, Lindqvist Y. Three-dimensional structures of glycolate oxidase with bound active-site inhibitors. Protein Sci. 1997;6:1009–1015. doi: 10.1002/pro.5560060506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatti DL, Palfey BA, Lah MS, Entsch B, Massey V, Ballou DP, Ludwig ML. The mobile flavin of 4-OH benzoate hydroxylase. Science. 1994;266:110–114. doi: 10.1126/science.7939628. [DOI] [PubMed] [Google Scholar]
- 34.Hall LH, Orchard BJ, Tripathy SK. The structure and properties of flavins: Molecular orbital study based on totally optimized geometries. I. Molecular geometry investigations. International Journal of Quantum Chemistry. 1987;31:195–216. [Google Scholar]
- 35.Diep Le KH, Lederer F. Amino acid sequence of long chain alpha-hydroxy acid oxidase from rat kidney, a member of the family of FMN-dependent alpha-hydroxy acid-oxidizing enzymes. J Biol Chem. 1991;266:20877–20881. [PubMed] [Google Scholar]
- 36.Mowat CG, Wehenkel A, Green AJ, Walkinshaw MD, Reid GA, Chapman SK. Altered substrate specificity in flavocytochrome b2: structural insights into the mechanism of L-lactate dehydrogenation. Biochemistry. 2004;43:9519–9526. doi: 10.1021/bi049263m. [DOI] [PubMed] [Google Scholar]
- 37.Binda C, Angelini R, Federico R, Ascenzi P, Mattevi A. Structural bases for inhibitor binding and catalysis in polyamine oxidase. Biochemistry. 2001;40:2766–2776. doi: 10.1021/bi002751j. [DOI] [PubMed] [Google Scholar]
- 38.Merenyi G, Lind J. Chemistry of peroxidic tetrahedral intermediates of flavin. Journal of American Chemical Society. 1991;113:3146–3153. [Google Scholar]
- 39.Otwinowski Z, Minor W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. In: Carter CWJ, Sweet RM, editors. Methods in Enzymology. Vol. 276. Academic Press; Boston: 1997. pp. 307–325. [DOI] [PubMed] [Google Scholar]
- 40.Collaborative Computational Project N. The CCP4 suite: programs for protein crystallography. Acta Cryst D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 41.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallographica Section D-Biological Crystallography. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 42.Jones TA, Zou J-Y, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 43.Howlin B, Butler SA, Moss DS, Harris GW, Driessen HPC. TLSANL: TLS parameter-analysis program for segmented anisotropic refinement of macromolecular structures. J Appl Crystallogr. 1993;26:622–624. [Google Scholar]
- 44.Lamzin VS, Perrakis A, Wilson KS. In: International Tables for Crystallography. Rossmann M, Arnold E, editors. F. Kluwer Academic Publishers; Dordrecht: 2001. pp. 721–722. [Google Scholar]
- 45.Shan SO, Herschlag D. The change in hydrogen bond strength accompanying charge rearrangement: implications for enzymatic catalysis. Proc Natl Acad Sci USA. 1993;93:14474–14479. doi: 10.1073/pnas.93.25.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]


