Abstract
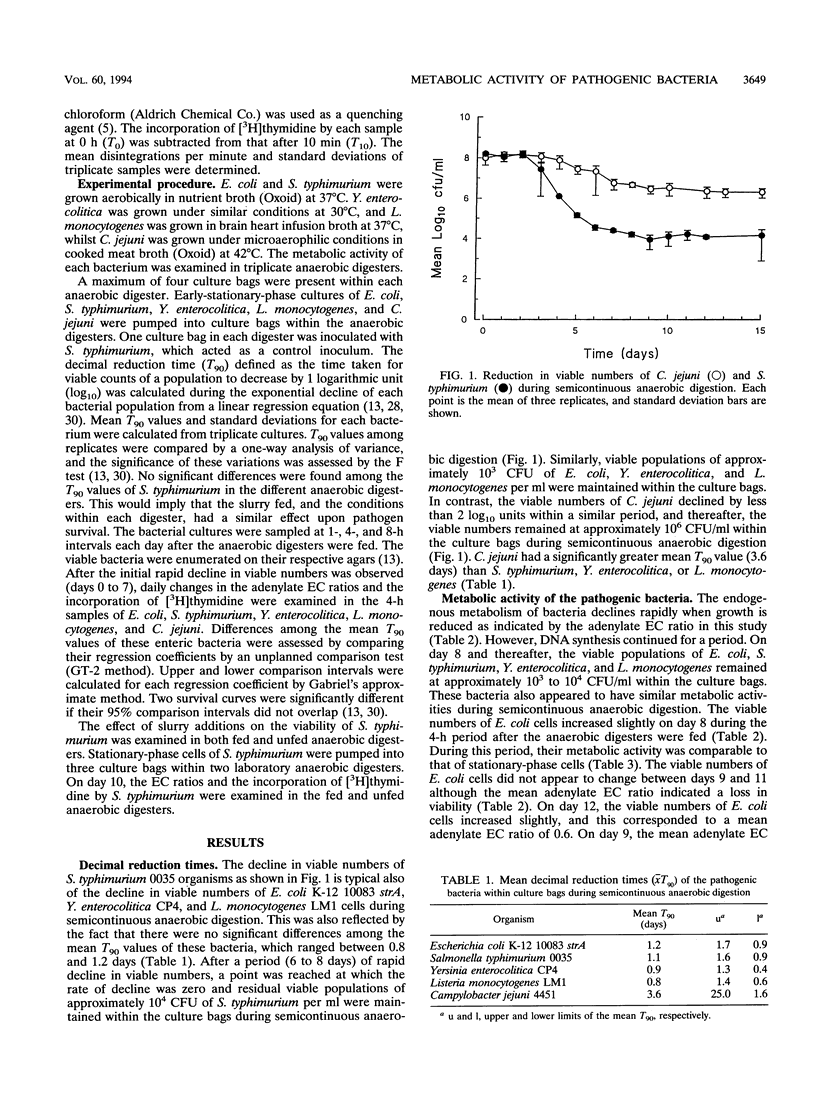
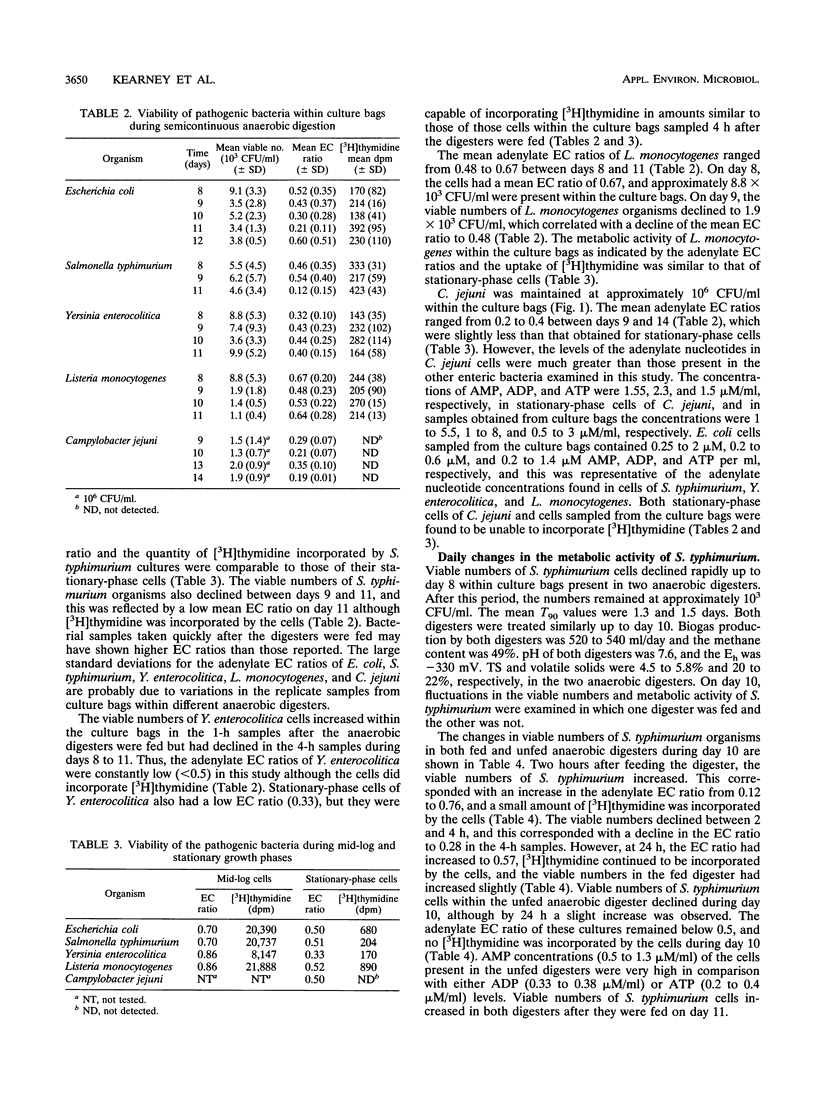
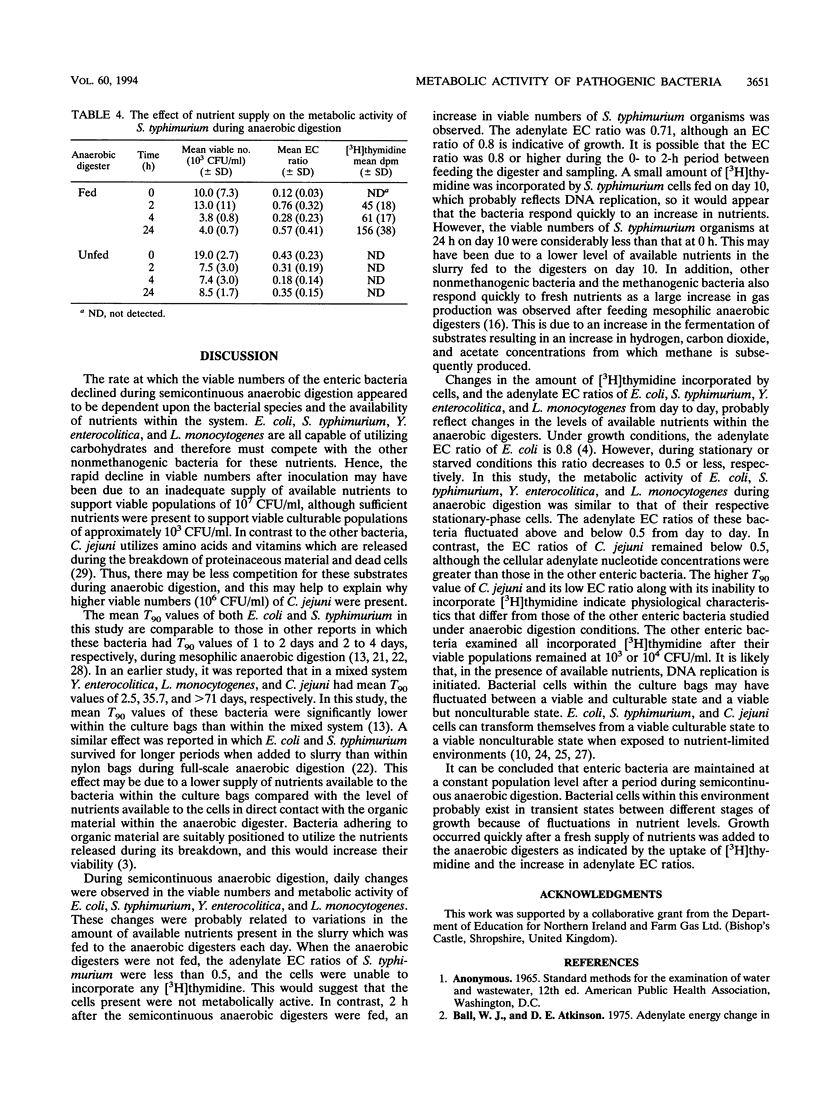
In natural environments such as anaerobic digesters, bacteria are frequently subjected to the stress of nutrient fluxes because of the continual changes in the flow of nutrients, and to survive, they must be capable of adapting readily to nutrient changes. In this study, the metabolic activities of Escherichia coli, Salmonella typhimurium, Yersinia enterocolitica, Listeria monocytogenes, and Campylobacter jejuni were studied within culture bags (Versapor-200 filters, 0.22-microns pore size) in laboratory anaerobic digesters. The metabolic activity of these bacteria was indicated by their adenylate energy charge (EC) ratios and their ability to incorporate [3H]thymidine, which was related to the respective changes in viable numbers within the culture bags during anaerobic digestion. Fluctuations in the adenylate EC ratios, the uptake of [3H]thymidine, and the viable numbers of E. coli, S. typhimurium, Y. enterocolitica, and L. monocytogenes cells were probably due to constant changes in the amount of available nutrients within the anaerobic digesters. The viability of S. typhimurium increased quickly after a fresh supply of nutrients was added to the system as indicated by the uptake of [3H]thymidine and an increase in the adenylate EC ratios. The viable numbers of E. coli, S. typhimurium, Y. enterocolitica, and L. monocytogenes organisms declined rapidly from 10(7) to 10(8) CFU/ml to 10(3) to 10(4) CFU/ml and remained at this level for an indefinite period. The decimal reduction time calculated during the period of exponential decline ranged from 0.8 to 1.2 days for these bacteria. C. jejuni had the greatest mean decimal reduction time value (3.6 days).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Dawes E. A. Stress of unbalanced growth and starvation in micro-organisms. Soc Appl Bacteriol Symp Ser. 1984;(12):19–43. [PubMed] [Google Scholar]
- Grimes D. J., Atwell R. W., Brayton P. R., Palmer L. M., Rollins D. M., Roszak D. B., Singleton F. L., Tamplin M. L., Colwell R. R. The fate of enteric pathogenic bacteria in estuarine and marine environments. Microbiol Sci. 1986 Nov;3(11):324–329. [PubMed] [Google Scholar]
- Jones D. M., Sutcliffe E. M., Curry A. Recovery of viable but non-culturable Campylobacter jejuni. J Gen Microbiol. 1991 Oct;137(10):2477–2482. doi: 10.1099/00221287-137-10-2477. [DOI] [PubMed] [Google Scholar]
- Kearney T. E., Larkin M. J., Levett P. N. The effect of slurry storage and anaerobic digestion on survival of pathogenic bacteria. J Appl Bacteriol. 1993 Jan;74(1):86–93. doi: 10.1111/j.1365-2672.1993.tb03000.x. [DOI] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Mackie R. I., Bryant M. P. Metabolic Activity of Fatty Acid-Oxidizing Bacteria and the Contribution of Acetate, Propionate, Butyrate, and CO(2) to Methanogenesis in Cattle Waste at 40 and 60 degrees C. Appl Environ Microbiol. 1981 Jun;41(6):1363–1373. doi: 10.1128/aem.41.6.1363-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- Rhodes M. W., Kator H. Survival of Escherichia coli and Salmonella spp. in estuarine environments. Appl Environ Microbiol. 1988 Dec;54(12):2902–2907. doi: 10.1128/aem.54.12.2902-2907.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Smibert R. M. The genus Campylobacter. Annu Rev Microbiol. 1978;32:673–709. doi: 10.1146/annurev.mi.32.100178.003325. [DOI] [PubMed] [Google Scholar]


