Abstract
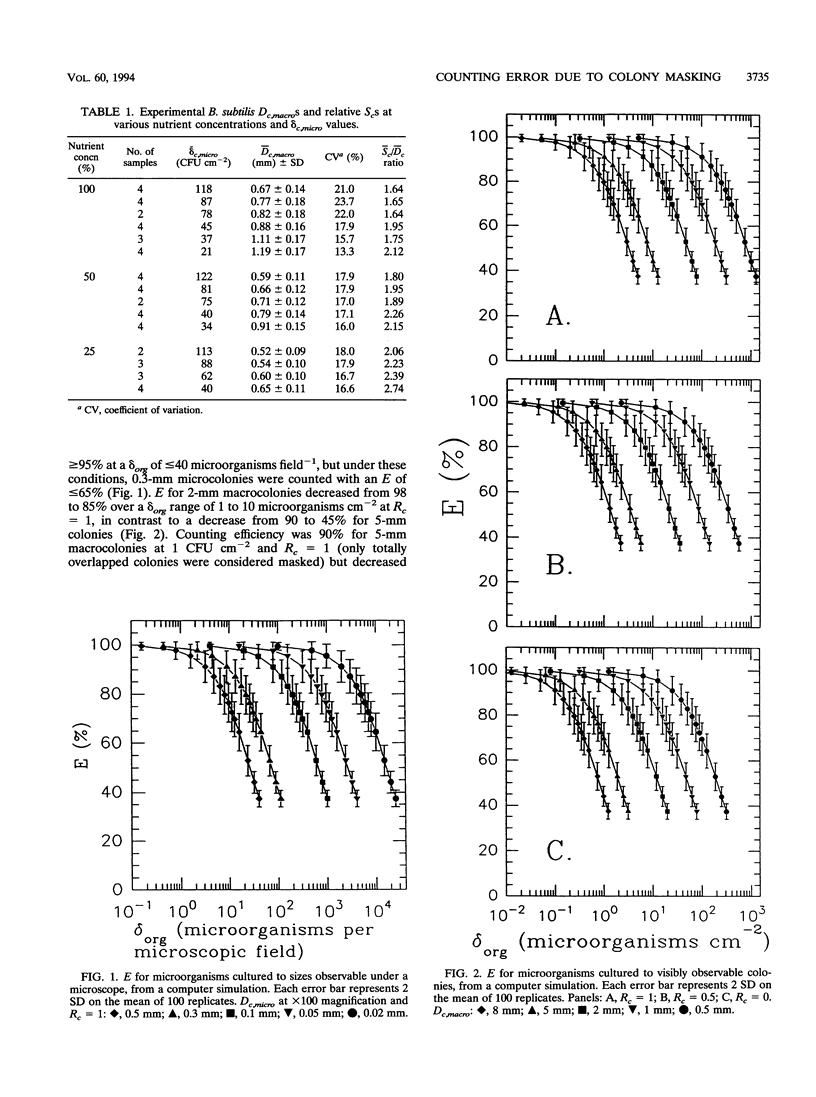
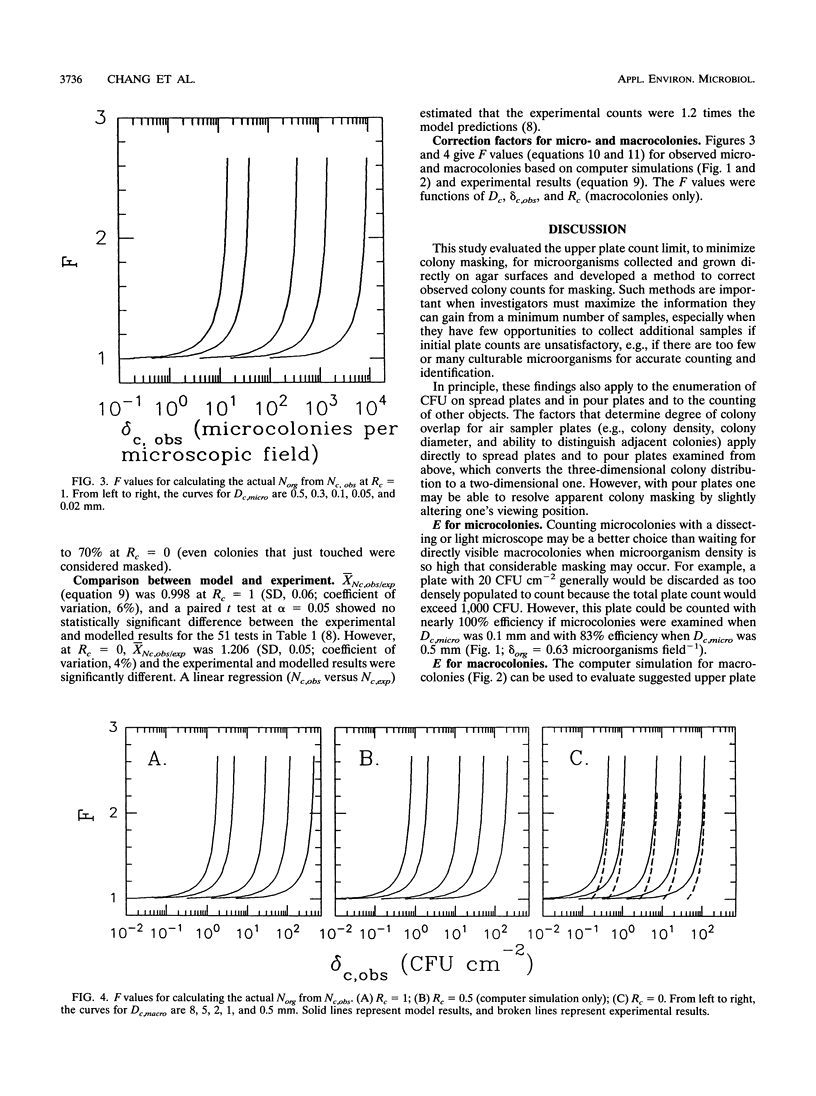
Colony counting error due to indistinguishable colony overlap (i.e., masking) was evaluated theoretically and experimentally. A theoretical model to predict colony masking was used to determine colony counting efficiency by Monte Carlo computer simulation of microorganism collection and development into CFU. The computer simulation was verified experimentally by collecting aerosolized Bacillus subtilis spores and examining micro- and macroscopic colonies. Colony counting efficiency decreased (i) with increasing density of collected culturable microorganisms, (ii) with increasing colony size, and (iii) with decreasing ability of an observation system to distinguish adjacent colonies as separate units. Counting efficiency for 2-mm colonies, at optimal resolution, decreased from 98 to 85% when colony density increased from 1 to 10 microorganisms cm-2, in contrast to an efficiency decrease from 90 to 45% for 5-mm colonies. No statistically significant difference (alpha = 0.05) between experimental and theoretical results was found when colony shape was used to estimate the number of individual colonies in a CFU. Experimental colony counts were 1.2 times simulation estimates when colony shape was not considered, because of nonuniformity of actual colony size and the better discrimination ability of the human eye relative to the model. Colony surface densities associated with high counting accuracy were compared with recommended upper plate count limits and found to depend on colony size and an observation system's ability to identify overlapped colonies. Correction factors were developed to estimate the actual number of collected microorganisms from observed colony counts.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMITAGE P. An overlap problem arising in particle counting. Biometrika. 1949 Dec;36(3-4):257–266. [PubMed] [Google Scholar]
- Beuchat L. R. Media for detecting and enumerating yeasts and moulds. Int J Food Microbiol. 1992 Oct;17(2):145–158. doi: 10.1016/0168-1605(92)90112-g. [DOI] [PubMed] [Google Scholar]
- Breed R. S., Dotterrer W. D. The Number of Colonies Allowable on Satisfactory Agar Plates. J Bacteriol. 1916 May;1(3):321–331. doi: 10.1128/jb.1.3.321-331.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S., Rylander R., Larsson L. Airborne bacteria, endotoxin and fungi in dust in poultry and swine confinement buildings. Am Ind Hyg Assoc J. 1983 Jul;44(7):537–541. doi: 10.1080/15298668391405265. [DOI] [PubMed] [Google Scholar]
- Donham K. J., Rubino M., Thedell T. D., Kammermeyer J. Potential health hazards to agricultural workers in swine confinement buildings. J Occup Med. 1977 Jun;19(6):383–387. doi: 10.1097/00043764-197706000-00004. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz J. Exposure to dust-borne bacteria in agriculture. I. Environmental studies. Arch Environ Health. 1978 Sep-Oct;33(5):250–259. doi: 10.1080/00039896.1978.10667344. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz J., Jabłoński L., Olenchock S. A. Occupational biohazards: a review. Am J Ind Med. 1988;14(5):605–623. doi: 10.1002/ajim.4700140511. [DOI] [PubMed] [Google Scholar]
- Fox B. C., Chamberlin L., Kulich P., Rae E. J., Webster L. R. Heavy contamination of operating room air by Penicillium species: identification of the source and attempts at decontamination. Am J Infect Control. 1990 Oct;18(5):300–306. doi: 10.1016/0196-6553(90)90229-l. [DOI] [PubMed] [Google Scholar]
- Howes D. W., Fazekas de St Groth Overlap and the errors of plaque counting. II.the bias of the variance and the concealment of errors. J Hyg (Lond) 1969 Jun;67(2):335–342. doi: 10.1017/s0022172400041735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes D. W. Overlap and the errors of plaque counting. I. The overlap biases of observed counts and their correction. J Hyg (Lond) 1969 Jun;67(2):317–334. doi: 10.1017/s0022172400041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juozaitis A., Willeke K., Grinshpun S. A., Donnelly J. Impaction onto a Glass Slide or Agar versus Impingement into a Liquid for the Collection and Recovery of Airborne Microorganisms. Appl Environ Microbiol. 1994 Mar;60(3):861–870. doi: 10.1128/aem.60.3.861-870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenline P. A., Scarpino P. V. Bacterial air pollution from sewage treatment plants. Am Ind Hyg Assoc J. 1972 May;33(5):346–352. doi: 10.1080/0002889728506659. [DOI] [PubMed] [Google Scholar]
- Knight G. Overlap problems in counting fibers. Am Ind Hyg Assoc J. 1975 Feb;36(2):113–114. doi: 10.1080/0002889758507218. [DOI] [PubMed] [Google Scholar]
- Kotimaa M. H., Husman K. H., Terho E. O., Mustonen M. H. Airborne molds and actinomycetes in the work environment of farmer's lung patients in Finland. Scand J Work Environ Health. 1984 Apr;10(2):115–119. doi: 10.5271/sjweh.2356. [DOI] [PubMed] [Google Scholar]
- Lacey J., Crook B. Fungal and actinomycete spores as pollutants of the workplace and occupational allergens. Ann Occup Hyg. 1988;32(4):515–533. doi: 10.1093/annhyg/32.4.515. [DOI] [PubMed] [Google Scholar]
- Lenhart S. W., Olenchock S. A., Cole E. C. Viable sampling for airborne bacteria in a poultry processing plant. J Toxicol Environ Health. 1982 Oct-Nov;10(4-5):613–619. doi: 10.1080/15287398209530280. [DOI] [PubMed] [Google Scholar]
- Logan N. A., Berkeley R. C. Identification of Bacillus strains using the API system. J Gen Microbiol. 1984 Jul;130(7):1871–1882. doi: 10.1099/00221287-130-7-1871. [DOI] [PubMed] [Google Scholar]
- Schuette W. H., Chen S. S., Occhipinti S. J., Mujagic H. S., Shackney S. E. Automated radioautographic grain counting. Correction for grain overlap. Cell Tissue Kinet. 1983 May;16(3):221–227. [PubMed] [Google Scholar]
- Smid T., Heederik D., Mensink G., Houba R., Boleij J. S. Exposure to dust, endotoxins, and fungi in the animal feed industry. Am Ind Hyg Assoc J. 1992 Jun;53(6):362–368. doi: 10.1080/15298669291359780. [DOI] [PubMed] [Google Scholar]
- Till J. E. Overlap error in counts of splenic colonies. Ser Haematol. 1972;5(2):5–14. [PubMed] [Google Scholar]
- VAN Soestbergen A. A., Lee C. H. Pour plates or streak plates? Appl Microbiol. 1969 Dec;18(6):1092–1093. doi: 10.1128/am.18.6.1092-1093.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znojil V., Necas E. The spleen colony technique. I. Correction for the overlap effect and sources of error in CFU-s determination. Cell Tissue Kinet. 1988 Mar;21(2):65–72. doi: 10.1111/j.1365-2184.1988.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Znojil V., Necas E. The spleen colony technique. II. Errors in estimation of the fraction of CFU-s synthesizing DNA. Cell Tissue Kinet. 1988 May;21(3):143–147. doi: 10.1111/j.1365-2184.1988.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Znojil V., Necas E. The spleen colony technique. III. Comparison of the overlap effect and of errors in CFU-s determination and the [3H]-thymidine suicide data for several strains of mice. Cell Tissue Kinet. 1988 May;21(3):149–157. doi: 10.1111/j.1365-2184.1988.tb00853.x. [DOI] [PubMed] [Google Scholar]


