Abstract
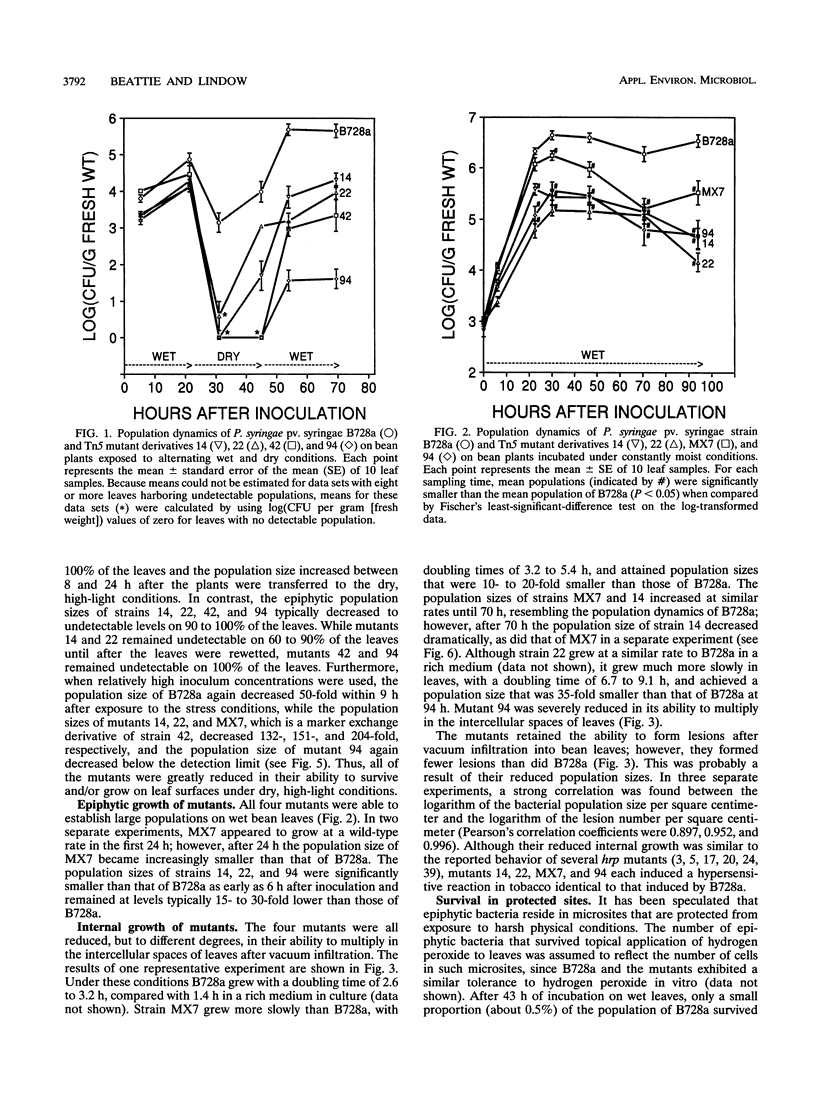
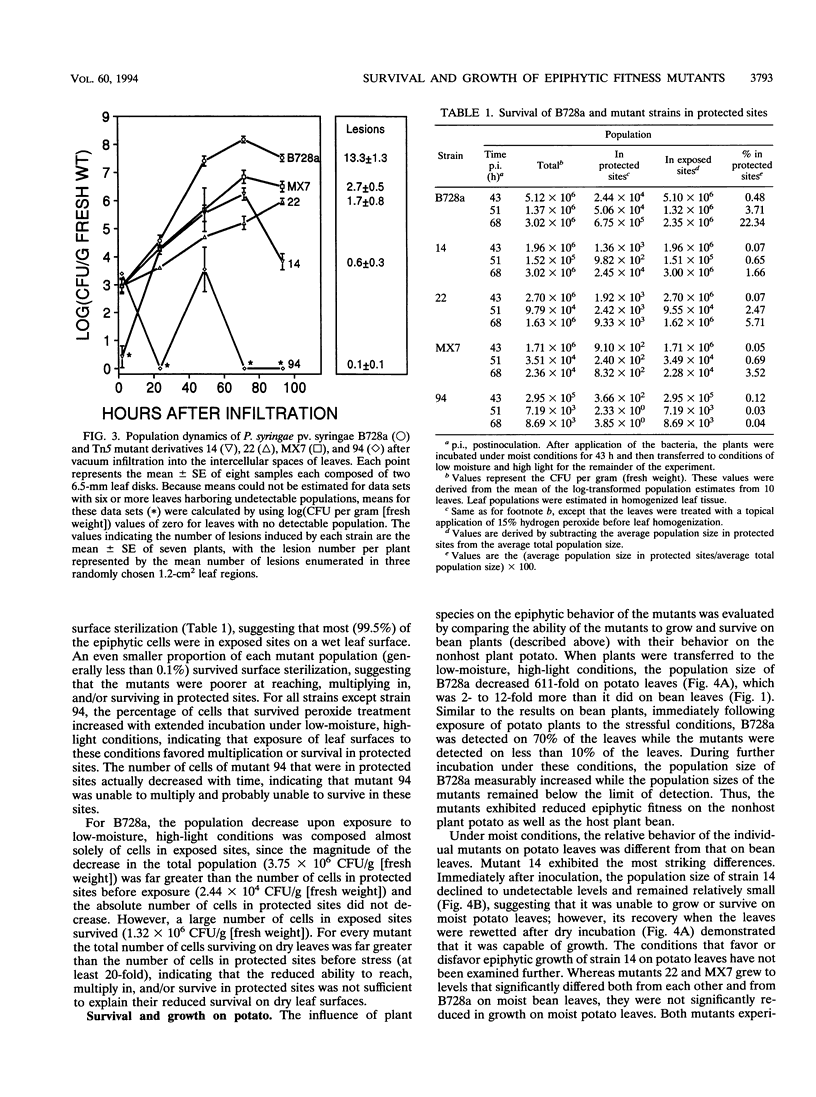
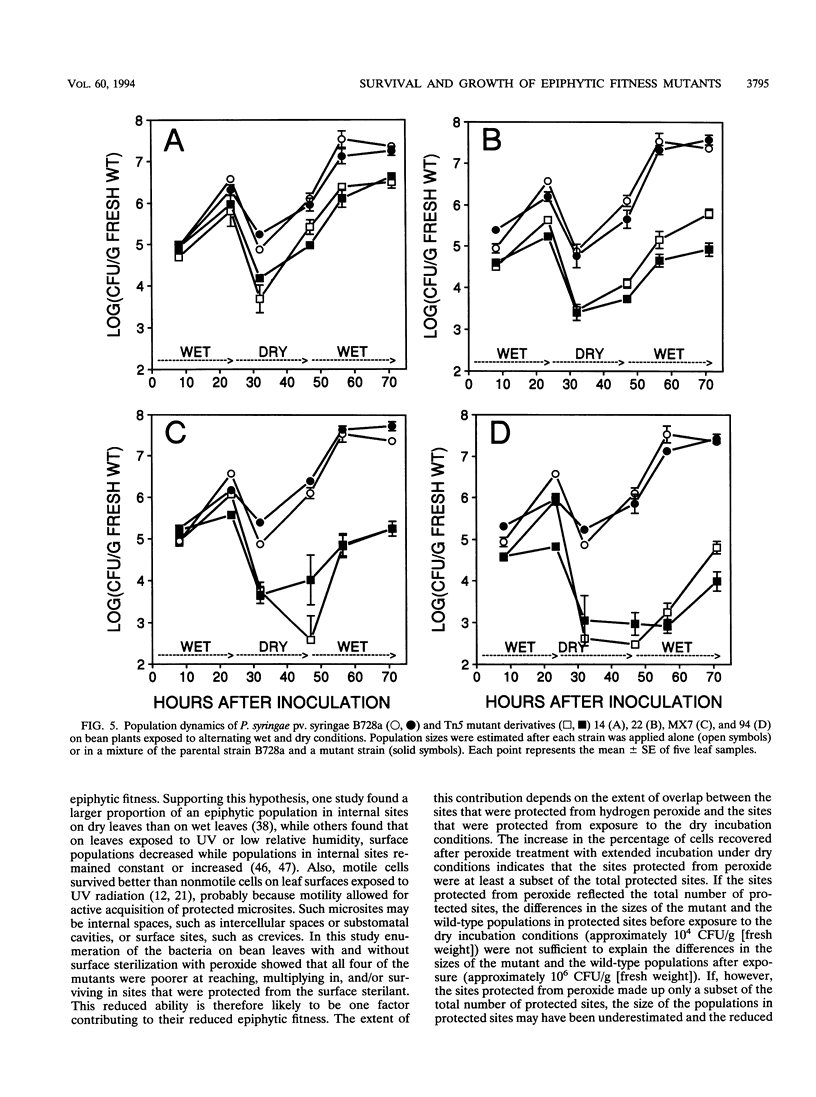
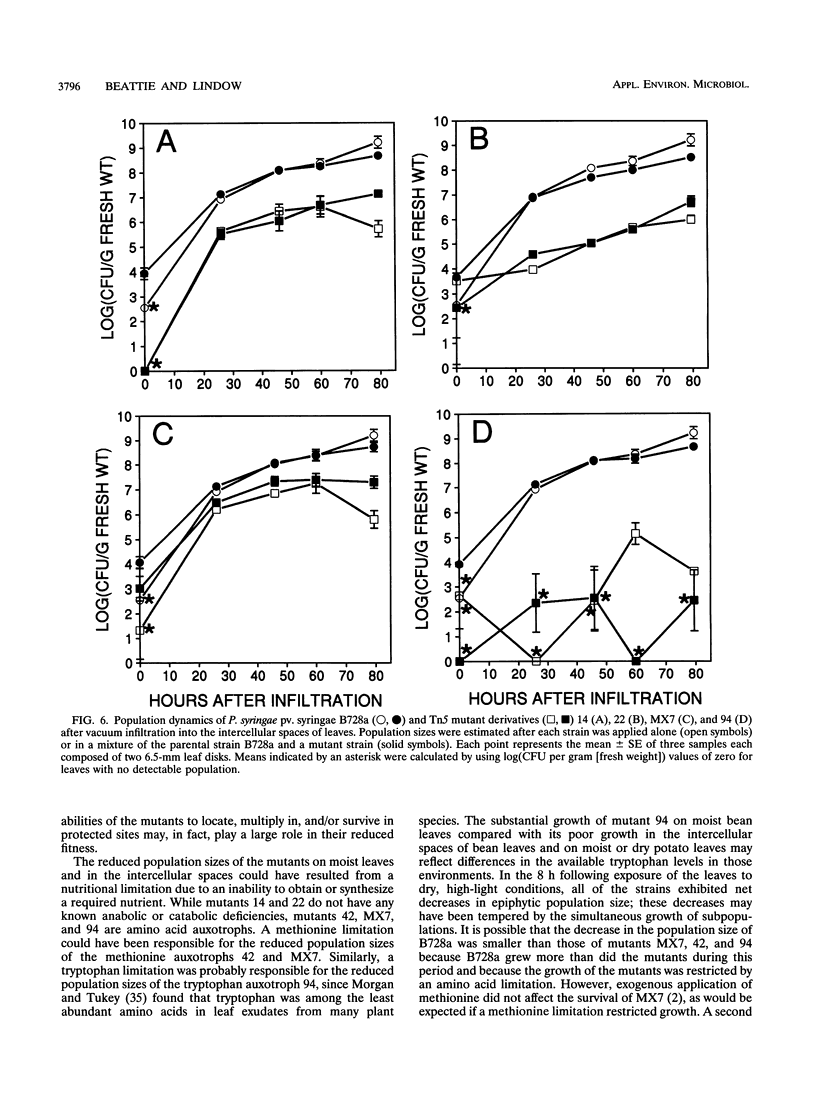
Among 82 epiphytic fitness mutants of a Pseudomonas syringae pv. syringae strain that were characterized in a previous study, 4 mutants were particularly intolerant of the stresses associated with dry leaf surfaces. These four mutants each exhibited distinctive behaviors when inoculated onto and into plant leaves. For example, while none showed measurable growth on dry potato leaf surfaces, they grew to different population sizes in the intercellular spaces of bean leaves and on dry bean leaf surfaces, and one mutant appeared incapable of growth in both environments although it grew well on moist bean leaves. The presence of the parental strain did not influence the survival of the mutants immediately following exposure of leaves to dry, high-light incubation conditions, suggesting that the reduced survival of the mutants did not result from an inability to produce extracellular factors in planta. On moist bean leaves that were colonized by either a mutant or the wild type, the proportion of the total epiphytic population that was located in sites protected from a surface sterilant was smaller for the mutants than for the wild type, indicating that the mutants were reduced in their ability to locate, multiply in, and/or survive in such protected sites. This reduced ability was only one of possibly several factors contributing to the reduced epiphytic fitness of each mutant. Their reduced fitness was not specific to the host plant bean, since they also exhibited reduced fitness on the nonhost plant potato; the functions altered in these strains are thus of interest for their contribution to the general fitness of bacterial epiphytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie G. A., Lindow S. E. Comparison of the Behavior of Epiphytic Fitness Mutants of Pseudomonas syringae under Controlled and Field Conditions. Appl Environ Microbiol. 1994 Oct;60(10):3799–3808. doi: 10.1128/aem.60.10.3799-3808.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefele D. M., Lindow S. E. Flagellar Motility Confers Epiphytic Fitness Advantages upon Pseudomonas syringae. Appl Environ Microbiol. 1987 Oct;53(10):2528–2533. doi: 10.1128/aem.53.10.2528-2533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter H. L., Moore A. H. Iterative maximum-likelihood estimation of the parameters of normal populations from singly and doubly censored samples. Biometrika. 1966 Jun;53(1):205–213. [PubMed] [Google Scholar]
- Hirano S. S., Nordheim E. V., Arny D. C., Upper C. D. Lognormal distribution of epiphytic bacterial populations on leaf surfaces. Appl Environ Microbiol. 1982 Sep;44(3):695–700. doi: 10.1128/aem.44.3.695-700.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kamoun S., Kado C. I. A plant-inducible gene of Xanthomonas campestris pv. campestris encodes an exocellular component required for growth in the host and hypersensitivity on nonhosts. J Bacteriol. 1990 Sep;172(9):5165–5172. doi: 10.1128/jb.172.9.5165-5172.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren P. B., Peet R. C., Panopoulos N. J. Gene cluster of Pseudomonas syringae pv. "phaseolicola" controls pathogenicity of bean plants and hypersensitivity of nonhost plants. J Bacteriol. 1986 Nov;168(2):512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Andersen G., Beattie G. A. Characteristics of Insertional Mutants of Pseudomonas syringae with Reduced Epiphytic Fitness. Appl Environ Microbiol. 1993 May;59(5):1593–1601. doi: 10.1128/aem.59.5.1593-1601.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Arny D. C., Upper C. D. Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiol. 1982 Oct;70(4):1084–1089. doi: 10.1104/pp.70.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E. Novel method for identifying bacterial mutants with reduced epiphytic fitness. Appl Environ Microbiol. 1993 May;59(5):1586–1592. doi: 10.1128/aem.59.5.1586-1592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. V., Tukey H. B. Characterization of Leachate from Plant Foliage. Plant Physiol. 1964 Jul;39(4):590–593. doi: 10.1104/pp.39.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme L. G., Mindrinos M. N., Panopoulos N. J. Genetic and transcriptional organization of the hrp cluster of Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1991 Jan;173(2):575–586. doi: 10.1128/jb.173.2.575-586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


