Abstract
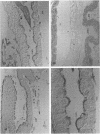
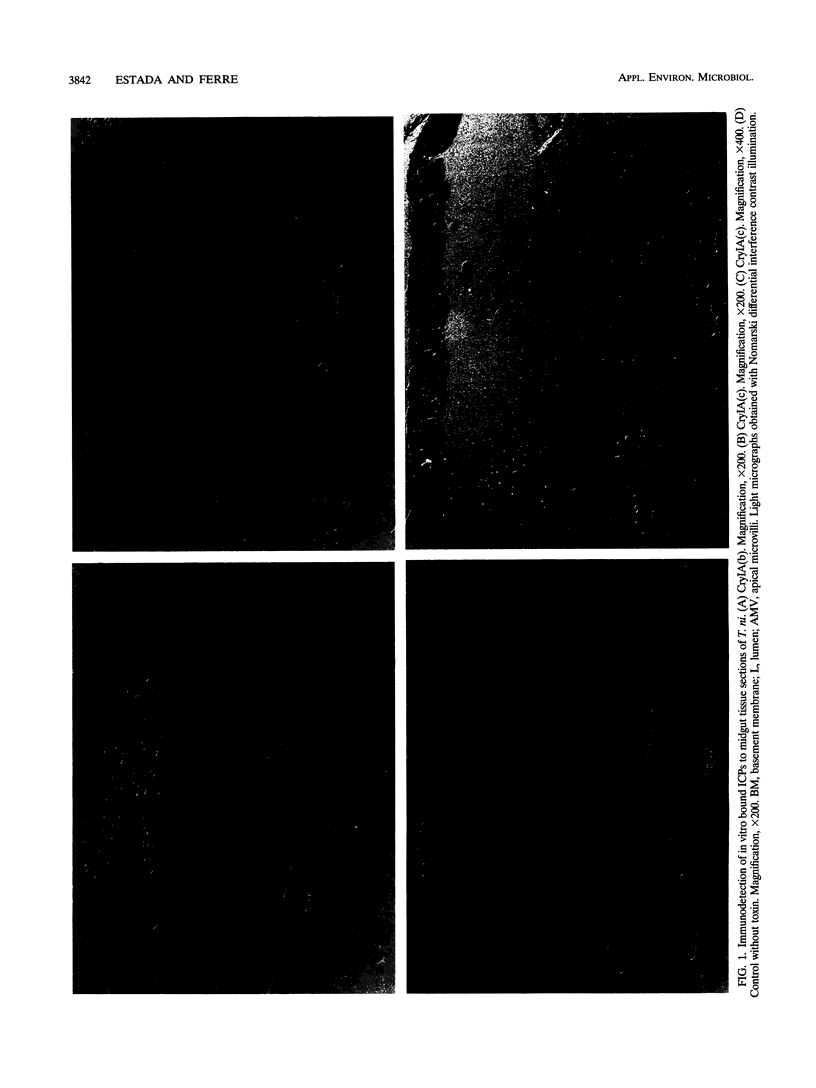
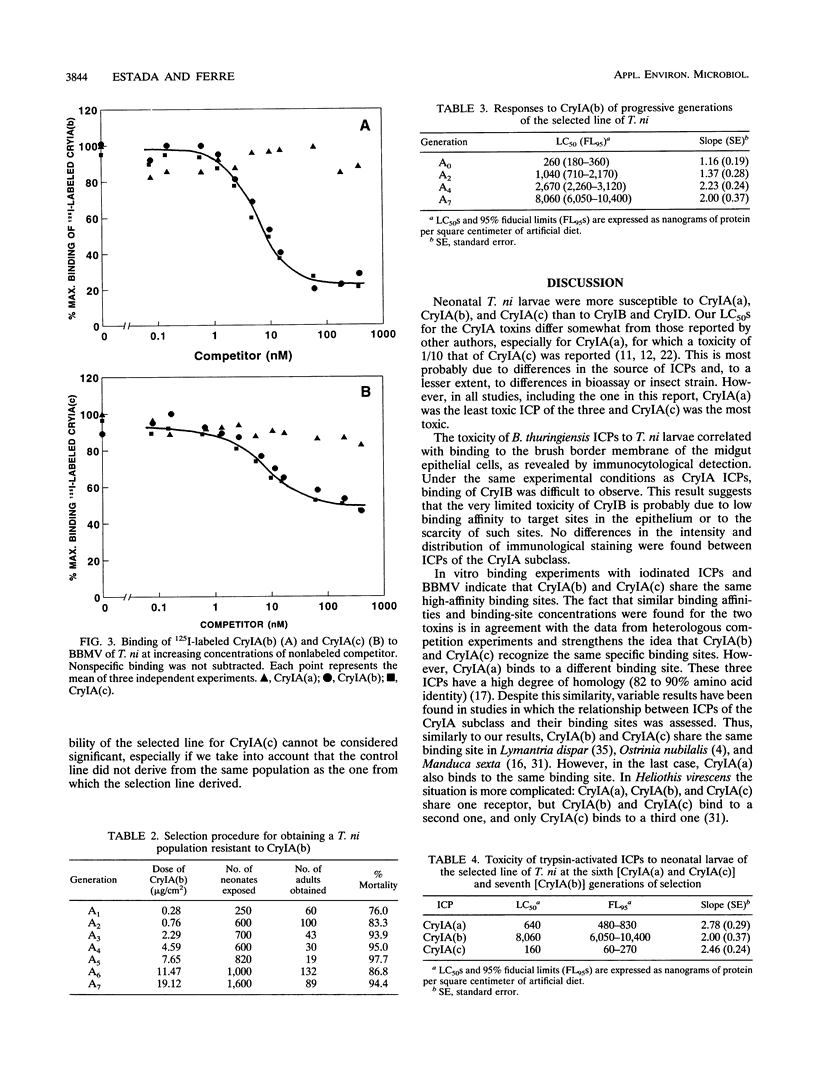
The susceptibility of Trichoplusia ni larvae to several Bacillus thuringiensis insecticidal crystal proteins (ICPs) was tested. Neonatal larvae proved to be susceptible to solubilized trypsin-treated CryIA(a), CryIA(b), and CryIA(c) (50% lethal concentrations [LC50s], 570, 480, and 320 ng/cm2, respectively) but showed little susceptibility to CryIB and CryID (LC50s, 5,640 and 2,530 ng/cm2, respectively). The toxicity of ICPs was correlated to binding to the epithelial brush border of the midgut, as revealed by immunocytochemical staining with monoclonal antibodies. In vitro binding experiments with iodinated ICPs and brush border membrane vesicles indicated that CryIA(b) and CryIA(c) share the same high-affinity binding site, whereas CryIA(a) binds to a different one. The affinities of CryIA(b) and CryIA(c) for the binding site were similar (Kd = 3.6 and 4.7 nM, respectively), and the mean binding-site concentration was 0.71 pmol/mg of vesicle protein. Selection of a population with increasing concentrations of CryIA(b) produced 31-fold resistance in seven generations. The realized heritability (h2) was 0.19. The increase of homozygosity (for resistance factors) as selection proceeded was reflected in the increase in the slopes of the dose-mortality curves. Resistance was specific for CryIA(b) and did not extend to CryIA(a) or even to CryIA(c). This result was not predicted by the binding-site model, in which CryIA(b) and CryIA(c) bind to the same high-affinity binding site. This result may suggest a more complicated relationship between in vitro binding of ICPs to specific sites in the epithelial membrane of the midgut and the in vivo toxic effect.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Denolf P., Jansens S., Peferoen M., Degheele D., Van Rie J. Two Different Bacillus thuringiensis Delta-Endotoxin Receptors in the Midgut Brush Border Membrane of the European Corn Borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae). Appl Environ Microbiol. 1993 Jun;59(6):1828–1837. doi: 10.1128/aem.59.6.1828-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denolf P., Jansens S., Van Houdt S., Peferoen M., Degheele D., Van Rie J. Biotinylation of Bacillus thuringiensis Insecticidal Crystal Proteins. Appl Environ Microbiol. 1993 Jun;59(6):1821–1827. doi: 10.1128/aem.59.6.1821-1827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré J., Real M. D., Van Rie J., Jansens S., Peferoen M. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge A. Z., Rivers D., Milne R., Dean D. H. Functional domains of Bacillus thuringiensis insecticidal crystal proteins. Refinement of Heliothis virescens and Trichoplusia ni specificity domains on CryIA(c). J Biol Chem. 1991 Sep 25;266(27):17954–17958. [PubMed] [Google Scholar]
- Ge A. Z., Shivarova N. I., Dean D. H. Location of the Bombyx mori specificity domain on a Bacillus thuringiensis delta-endotoxin protein. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4037–4041. doi: 10.1073/pnas.86.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S., Cowles E. A., Pietrantonio P. V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- Gould F., Martinez-Ramirez A., Anderson A., Ferre J., Silva F. J., Moar W. J. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7986–7990. doi: 10.1073/pnas.89.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C., Vanderbruggen H., Höfte H., Van Rie J., Jansens S., Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins is correlated with the presence of high-affinity binding sites in the brush border membrane of target insect midguts. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7844–7848. doi: 10.1073/pnas.85.21.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. D., Carroll J., Ellar D. J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature. 1991 Oct 31;353(6347):815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- MacIntosh S. C., Stone T. B., Jokerst R. S., Fuchs R. L. Binding of Bacillus thuringiensis proteins to a laboratory-selected line of Heliothis virescens. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8930–8933. doi: 10.1073/pnas.88.20.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughey W. H. Insect Resistance to the Biological Insecticide Bacillus thuringiensis. Science. 1985 Jul 12;229(4709):193–195. doi: 10.1126/science.229.4709.193. [DOI] [PubMed] [Google Scholar]
- Moar W. J., Masson L., Brousseau R., Trumble J. T. Toxicity to Spodoptera exigua and Trichoplusia ni of individual P1 protoxins and sporulated cultures of Bacillus thuringiensis subsp. kurstaki HD-1 and NRD-12. Appl Environ Microbiol. 1990 Aug;56(8):2480–2483. doi: 10.1128/aem.56.8.2480-2483.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Van Rie J., Jansens S., Höfte H., Degheele D., Van Mellaert H. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxins. Appl Environ Microbiol. 1990 May;56(5):1378–1385. doi: 10.1128/aem.56.5.1378-1385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie J., Jansens S., Höfte H., Degheele D., Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on the brush border membrane of the mid-gut of target insects. Eur J Biochem. 1989 Dec 8;186(1-2):239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- Van Rie J., McGaughey W. H., Johnson D. E., Barnett B. D., Van Mellaert H. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science. 1990 Jan 5;247(4938):72–74. doi: 10.1126/science.2294593. [DOI] [PubMed] [Google Scholar]
- Wolfersberger M. G. The toxicity of two Bacillus thuringiensis delta-endotoxins to gypsy moth larvae is inversely related to the affinity of binding sites on midgut brush border membranes for the toxins. Experientia. 1990 May 15;46(5):475–477. doi: 10.1007/BF01954236. [DOI] [PubMed] [Google Scholar]