Abstract
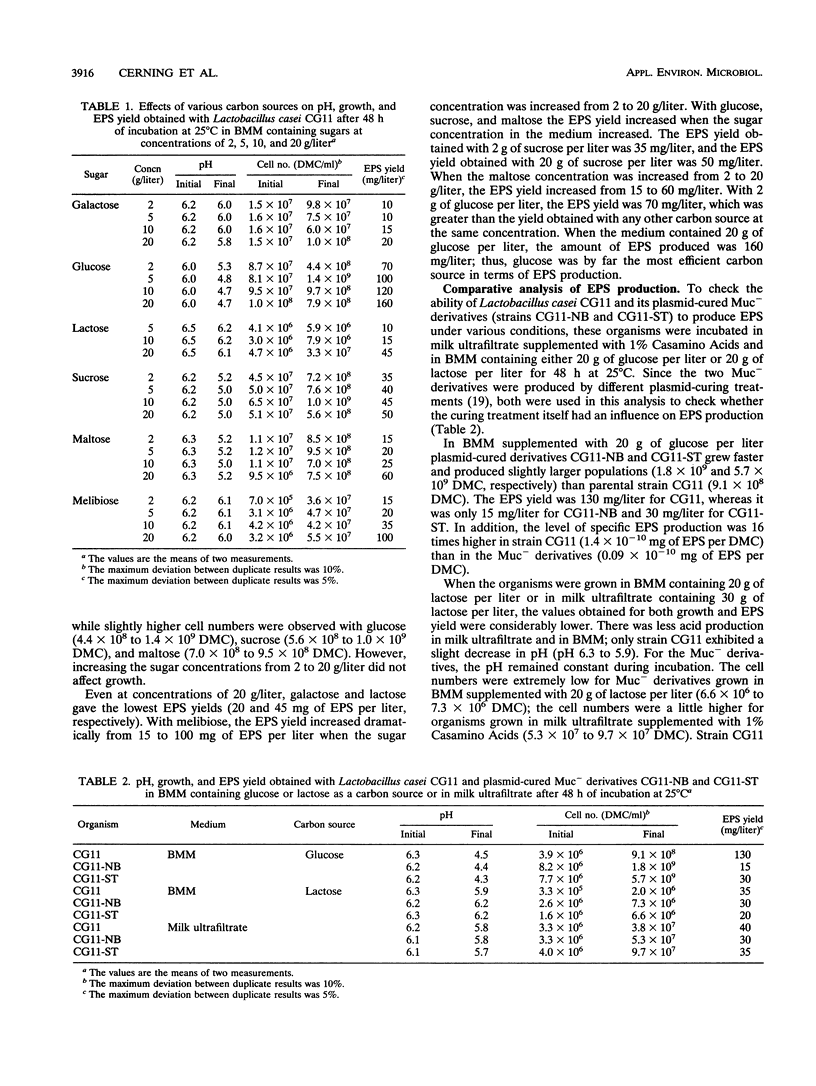
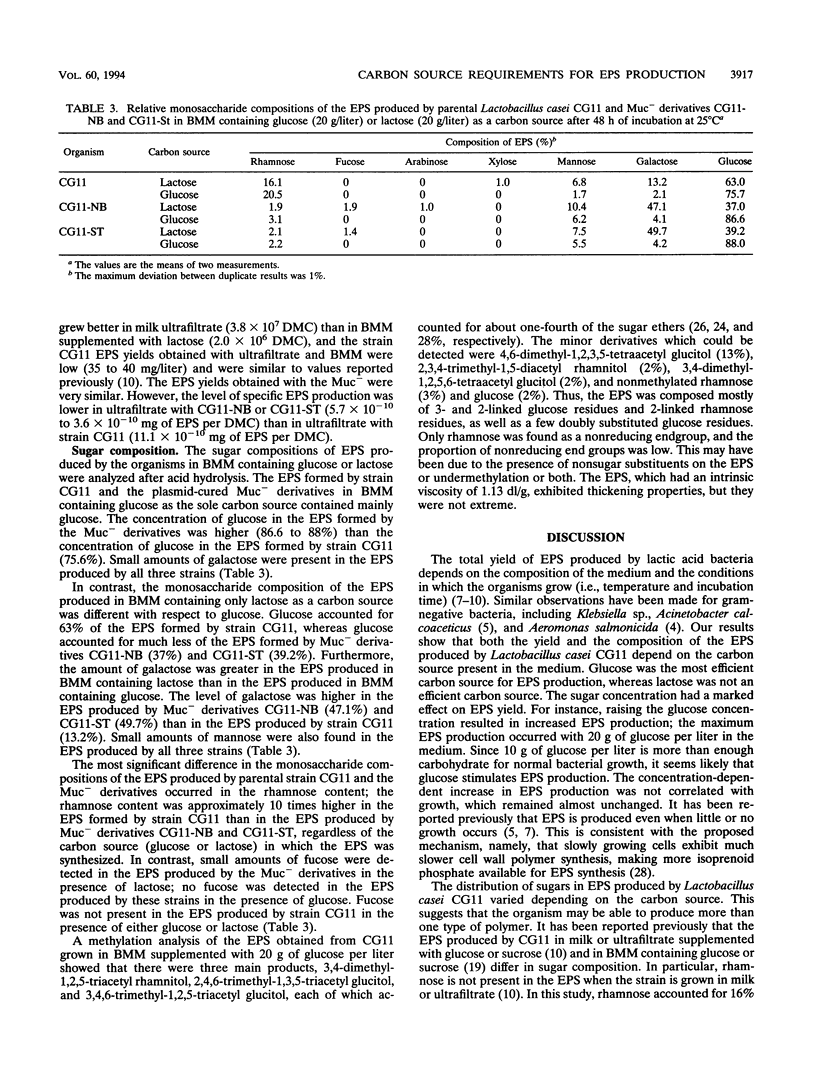
Exopolysaccharide production by Lactobacillus casei CG11 was studied in basal minimum medium containing various carbon sources (galactose, glucose, lactose, sucrose, maltose, melibiose) at concentrations of 2, 5, 10, and 20 g/liter. L. casei CG11 produced exopolysaccharides in basal minimum medium containing each of the sugars tested; lactose and galactose were the poorest carbon sources, and glucose was by far the most efficient carbon source. Sugar concentrations had a marked effect on polymer yield. Plasmid-cured Muc- derivatives grew better in the presence of glucose and attained slightly higher populations than the wild-type strain. The values obtained with lactose were considerably lower for both growth and exopolysaccharide yield. The level of specific polymer production per cell obtained with glucose was distinctively lower for Muc- derivatives than for the Muc+ strain. The polymer produced by L. casei CG11 in the presence of glucose was different from that formed in the presence of lactose. The polysaccharide produced by L. casei CG11 in basal minimum medium containing 20 g of glucose per liter had an intrinsic viscosity of 1.13 dl/g. It was rich in glucose (76%), which was present mostly as 2- or 3-linked residues along with some 2,3 doubly substituted glucose units, and in rhamnose (21%), which was present as 2-linked or terminal rhamnose; traces of mannose and galactose were also present.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelos M. A., Thibault J. F. Influence of the substituents of the carboxyl groups and of the rhamnose content on the solution properties and flexibility of pectins. Int J Biol Macromol. 1991 Apr;13(2):77–82. doi: 10.1016/0141-8130(91)90052-v. [DOI] [PubMed] [Google Scholar]
- Bonet R., Simon-Pujol M. D., Congregado F. Effects of nutrients on exopolysaccharide production and surface properties of Aeromonas salmonicida. Appl Environ Microbiol. 1993 Aug;59(8):2437–2441. doi: 10.1128/aem.59.8.2437-2441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan B. A., Linhardt R. J., Daniels L. Variation in composition and yield of exopolysaccharides produced by Klebsiella sp. strain K32 and Acinetobacter calcoaceticus BD4. Appl Environ Microbiol. 1986 Jun;51(6):1304–1308. doi: 10.1128/aem.51.6.1304-1308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerning J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):113–130. doi: 10.1111/j.1574-6968.1990.tb04883.x. [DOI] [PubMed] [Google Scholar]
- Doco T., Wieruszeski J. M., Fournet B., Carcano D., Ramos P., Loones A. Structure of an exocellular polysaccharide produced by Streptococcus thermophilus. Carbohydr Res. 1990 May 1;198(2):313–321. doi: 10.1016/0008-6215(90)84301-a. [DOI] [PubMed] [Google Scholar]
- Gruter M., Leeflang B. R., Kuiper J., Kamerling J. P., Vliegenthart J. F. Structural characterisation of the exopolysaccharide produced by Lactobacillus delbrückii subspecies bulgaricus rr grown in skimmed milk. Carbohydr Res. 1993 Feb 1;239:209–226. doi: 10.1016/0008-6215(93)84216-s. [DOI] [PubMed] [Google Scholar]
- Gruter M., Leeflang B. R., Kuiper J., Kamerling J. P., Vliegenthart J. F. Structure of the exopolysaccharide produced by Lactococcus lactis subspecies cremoris H414 grown in a defined medium or skimmed milk. Carbohydr Res. 1992 Jul 2;231:273–291. doi: 10.1016/0008-6215(92)84025-n. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Kojic M., Vujcic M., Banina A., Cocconcelli P., Cerning J., Topisirovic L. Analysis of exopolysaccharide production by Lactobacillus casei CG11, isolated from cheese. Appl Environ Microbiol. 1992 Dec;58(12):4086–4088. doi: 10.1128/aem.58.12.4086-4088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita T., Deguchi Y., Yajima M., Sakurai T., Yura T. Multiple nutritional requirements of lactobacilli: genetic lesions affecting amino acid biosynthetic pathways. J Bacteriol. 1981 Oct;148(1):64–71. doi: 10.1128/jb.148.1.64-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Hirota T., Toba T., Itoh T., Adachi S. Structure of the extracellular polysaccharide from slime-forming Lactococcus lactis subsp. cremoris SBT 0495. Carbohydr Res. 1992 Feb 7;224:245–253. doi: 10.1016/0008-6215(92)84110-e. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Bacterial exopolysaccharides. Adv Microb Physiol. 1972;8:143–213. doi: 10.1016/s0065-2911(08)60190-3. [DOI] [PubMed] [Google Scholar]


