Abstract
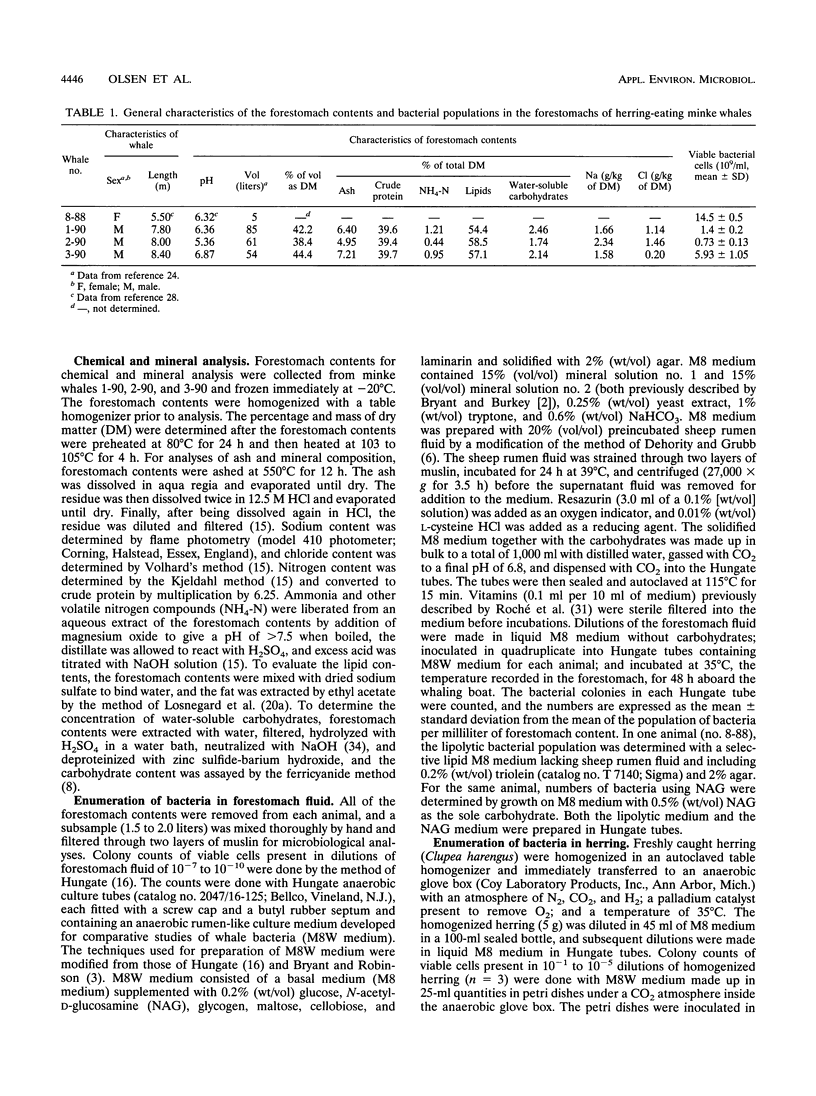
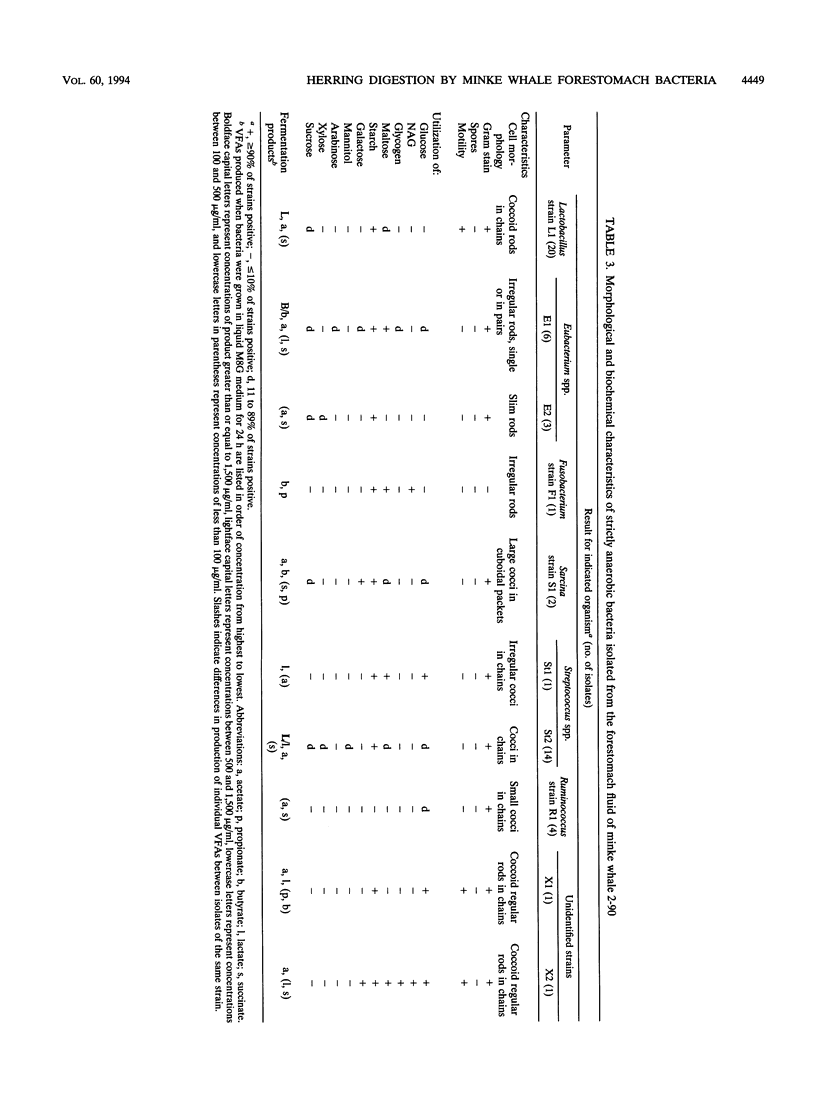
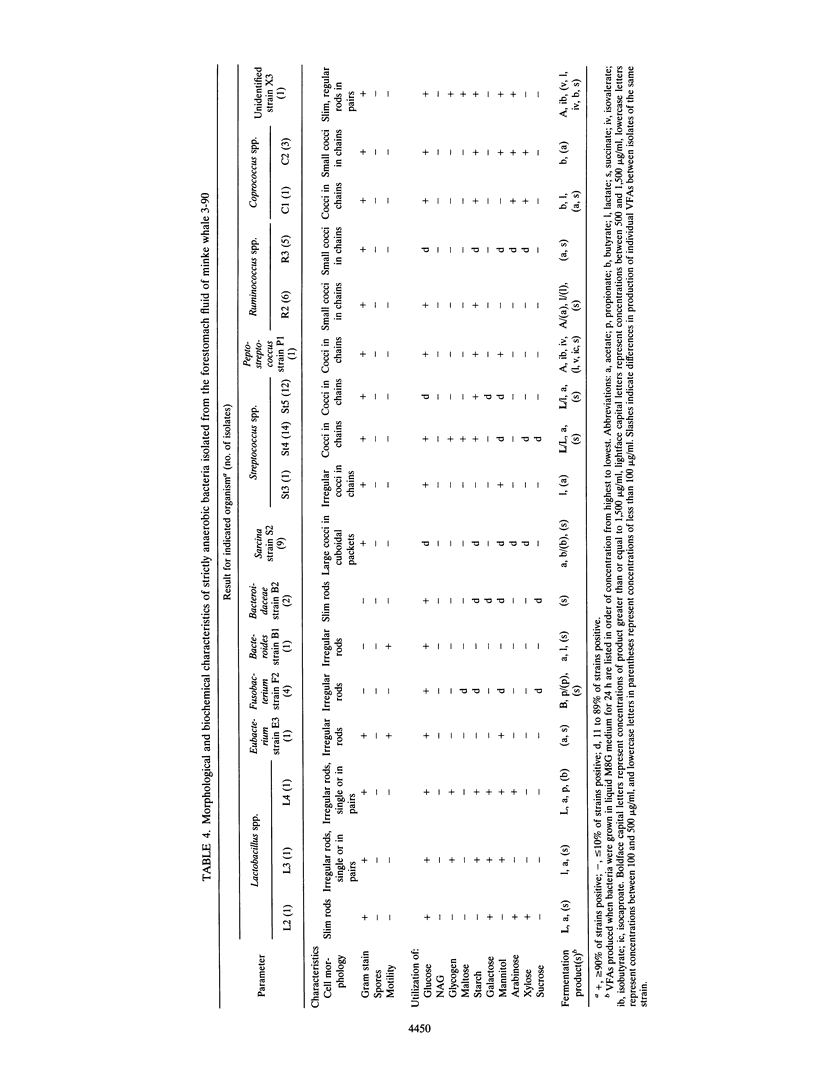
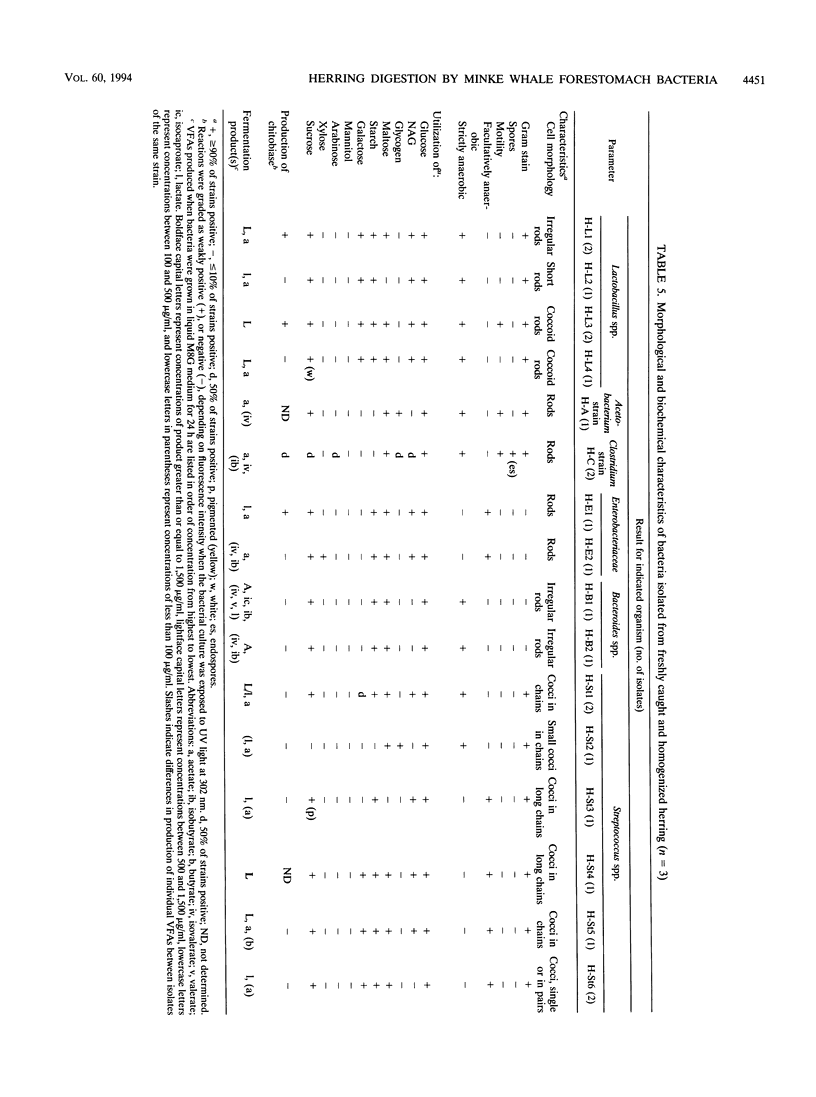
Northeastern Atlantic minke whales (Balaenoptera acutorostrata) have a multichambered stomach system which includes a nonglandular forestomach resembling that of ruminants. Bacteria from the forestomachs of herring-eating whales were enumerated and isolated in an anaerobic rumen-like culture medium (M8W medium). The total viable population of anaerobic bacteria ranged from 73 × 107 to 145 × 108/ml of forestomach fluid (n = 4). Lactobacillus spp. (19.7%), Streptococcus spp. (35.9%), and Ruminococcus spp. (12.8%) were the most common of the bacterial strains (n = 117) isolated by use of M8W medium from the forestomach fluid population of two minke whales. Most of the isolates stained gram positive (93.2%), 62.4% were cocci, and all strains were strictly anaerobic. The population of lipolytic bacteria in one animal, enumerated by use of a selective lipid medium, constituted 89.7% of the viable population. The total viable population of anaerobic bacteria in freshly caught and homogenized herring (Clupea harengus) ranged from 56.7 to 95.0 cells per gram of homogenized prey (n = 3) when M8W medium was used. Pediococcus spp. (30.6%) and Aerococcus spp. (25.0%) were most common of the bacterial strains (n = 72) isolated from the homogenized herring. Most of the bacterial strains were gram positive (80.6%), and 70.8% were cocci. Unlike the forestomach bacterial population, as many as 61.1% of the strains from the herring were facultatively anaerobic. All bacterial strains isolated from the prey had phenotypic patterns different from those of strains isolated from the dominant bacterial population in the forestomach, indicating that the forestomach microbiota is indigenous. Scanning electron microscopic examinations revealed large numbers of bacteria, surrounded by a glycocalyx, attached to partly digested food particles in the forestomach. These data support the hypothesis that symbiotic microbial digestion occurs in the forestomach and that the bacteria are indigenous to minke whales.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Dehority B. A., Grubb J. A. Basal medium for the selective enumeration of rumen bacteria utilizing specific energy sources. Appl Environ Microbiol. 1976 Nov;32(5):703–710. doi: 10.1128/aem.32.5.703-710.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig R. P., Staley J. T., Nerini M. K., Braham H. W. Baleen whales: preliminary evidence for forestomach microbial fermentation. Appl Environ Microbiol. 1984 Feb;47(2):421–423. doi: 10.1128/aem.47.2.421-423.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leedle J. A., Bryant M. P., Hespell R. B. Diurnal variations in bacterial numbers and fluid parameters in ruminal contents of animals fed low- or high-forage diets. Appl Environ Microbiol. 1982 Aug;44(2):402–412. doi: 10.1128/aem.44.2.402-412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordøy E. S., Sørmo W., Blix A. S. In vitro digestibility of different prey species of minke whales (Balaenoptera acutorostrata). Br J Nutr. 1993 Sep;70(2):485–489. doi: 10.1079/bjn19930142. [DOI] [PubMed] [Google Scholar]
- O'Brien M., Colwell R. R. A rapid test for chitinase activity that uses 4-methylumbelliferyl-N-acetyl-beta-D-glucosaminide. Appl Environ Microbiol. 1987 Jul;53(7):1718–1720. doi: 10.1128/aem.53.7.1718-1720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpin C. G., Mathiesen S. D., Greenwood Y., Blix A. S. Seasonal changes in the ruminal microflora of the high-arctic Svalbard reindeer (Rangifer tarandus platyrhynchus). Appl Environ Microbiol. 1985 Jul;50(1):144–151. doi: 10.1128/aem.50.1.144-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roché C., Albertyn H., van Gylswyk N. O., Kistner A. The growth response of cellulolytic acetate-utilizing and acetate-producing butyruvibrios to volatile fatty acids and other nutrients. J Gen Microbiol. 1973 Oct;78(2):253–260. doi: 10.1099/00221287-78-2-253. [DOI] [PubMed] [Google Scholar]
- Shewan J. M. THE STRICT ANAEROBES IN THE SLIME AND INTESTINES OF THE HADDOCK (GADUS AEGLEFINUS). J Bacteriol. 1938 Apr;35(4):397–407. doi: 10.1128/jb.35.4.397-407.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm A. R., Larsen H. Anaerobic fish spoilage by bacteria. I. Biochemical changes in herring extracts. J Appl Bacteriol. 1979 Jun;46(3):531–543. doi: 10.1111/j.1365-2672.1979.tb00852.x. [DOI] [PubMed] [Google Scholar]



