Abstract
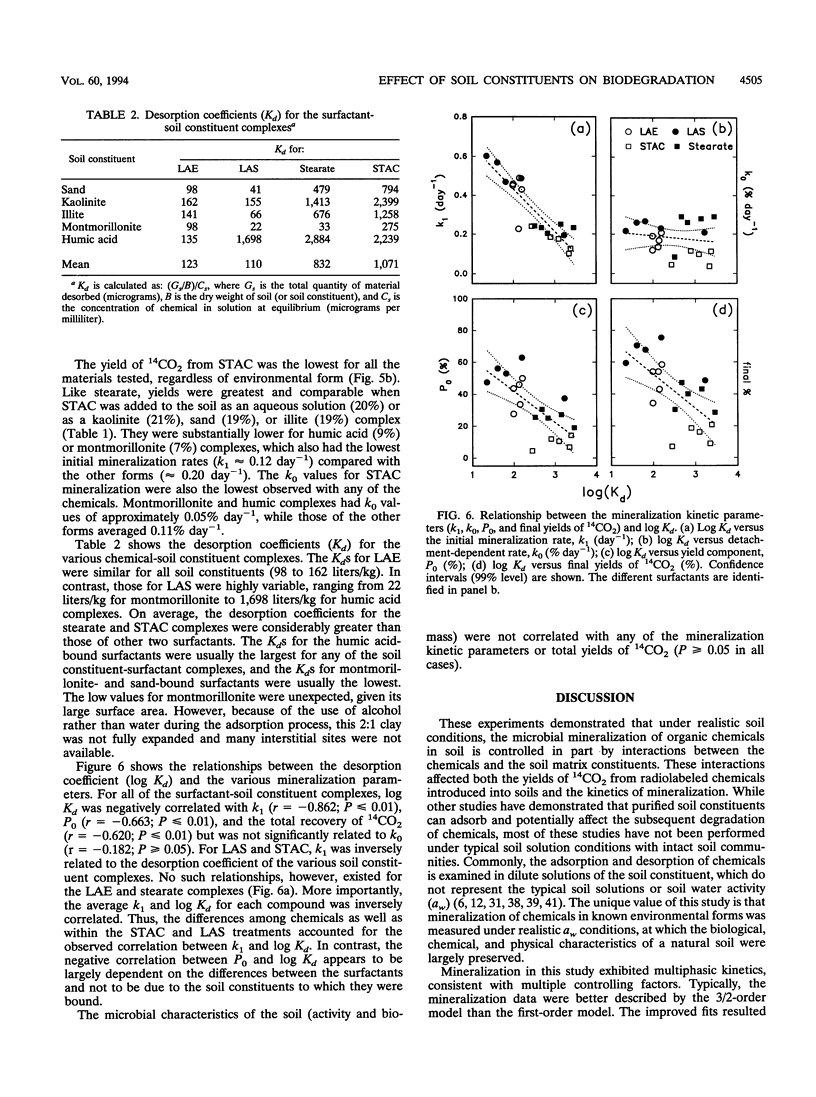
This research addressed the effect of mineral and organic soil constituents on the fate of organic compounds in soils. Specifically, it sought to determine how the associations between organic chemicals and different soil constituents affect their subsequent biodegradation in soil. Four 14C-labeled surfactants were aseptically adsorbed to montmorillonite, kaolinite, illite, sand, and humic acids. These complexes were mixed with a woodlot soil, and 14CO2 production was measured over time. The mineralization data were fitted to various production models by nonlinear regression, and a mixed (3/2)-order model was found to most accurately describe the mineralization patterns. Different mineralization patterns were observed as a function of the chemical and soil constituents. Surfactants that had been preadsorbed to sand or kaolinite usually showed similar mineralization kinetics to the control treatments, in which the surfactants were added to the soil as an aqueous solution. Surfactants that had been bound to illite or montmorillonite were typically degraded to lesser extents than the other forms, while surfactant-humic acid complexes were degraded more slowly than the other forms. The desorption coefficients (Kd) of the soil constituent-bound surfactants were negatively correlated with the initial rates of degradation (k1) and estimates of 14CO2 yield (Po) as well as actual total yields of 14CO2. However, there was no relationship between Kd and second-stage zero-order rates of mineralization (ko). Microbial community characteristics (biomass and activity) were not correlated with any of the mineralization kinetic parameters. Overall, this study showed that environmental form had a profound effect on the ultimate fate of biodegradable chemicals in soil. This form is defined by the physicochemical characteristics of the chemical, the composition and mineralogy of the soil, and the mode of entry of the chemical into the soil environment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunner W., Focht D. D. Deterministic three-half-order kinetic model for microbial degradation of added carbon substrates in soil. Appl Environ Microbiol. 1984 Jan;47(1):167–172. doi: 10.1128/aem.47.1.167-172.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay R. H., King G. M., Watling L. Efficacy of phospholipid analysis in determining microbial biomass in sediments. Appl Environ Microbiol. 1989 Nov;55(11):2888–2893. doi: 10.1128/aem.55.11.2888-2893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin W. F., Boyd S. A. Differential bioavailability of soil-sorbed naphthalene to two bacterial species. Appl Environ Microbiol. 1992 Apr;58(4):1142–1152. doi: 10.1128/aem.58.4.1142-1152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. S., Bartha R. Hydrolyzable and nonhydrolyzable 3,4-dichloroaniline-humus complexes and their respective rates of biodegradation. J Agric Food Chem. 1976 Jan-Feb;24(1):118–122. doi: 10.1021/jf60203a021. [DOI] [PubMed] [Google Scholar]
- Jacobsen C. S., Pedersen J. C. Mineralization of 2,4-dichlorophenoxyacetic acid (2,4-D) in soil inoculated with Pseudomonas cepacia DBO1(pRO101), Alcaligenes eutrophus AEO106(pRO101) and Alcaligenes eutrophus JMP134(pJP4): effects of inoculation level and substrate concentration. Biodegradation. 1991;2(4):253–263. doi: 10.1007/BF00114557. [DOI] [PubMed] [Google Scholar]
- McKinley V. L., Federle T. W., Vestal J. R. Effects of petroleum hydrocarbons on plant litter microbiota in an arctic lake. Appl Environ Microbiol. 1982 Jan;43(1):129–135. doi: 10.1128/aem.43.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogram A. V., Jessup R. E., Ou L. T., Rao P. S. Effects of sorption on biological degradation rates of (2,4-dichlorophenoxy) acetic acid in soils. Appl Environ Microbiol. 1985 Mar;49(3):582–587. doi: 10.1128/aem.49.3.582-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward T. E., Larson R. J. Biodegradation kinetics of linear alkylbenzene sulfonate in sludge-amended agricultural soils. Ecotoxicol Environ Saf. 1989 Feb;17(1):119–130. doi: 10.1016/0147-6513(89)90016-x. [DOI] [PubMed] [Google Scholar]
- al-Bashir B., Cseh T., Leduc R., Samson R. Effect of soil/contaminant interactions on the biodegradation of naphthalene in flooded soil under denitrifying conditions. Appl Microbiol Biotechnol. 1990 Dec;34(3):414–419. doi: 10.1007/BF00170071. [DOI] [PubMed] [Google Scholar]


