Abstract
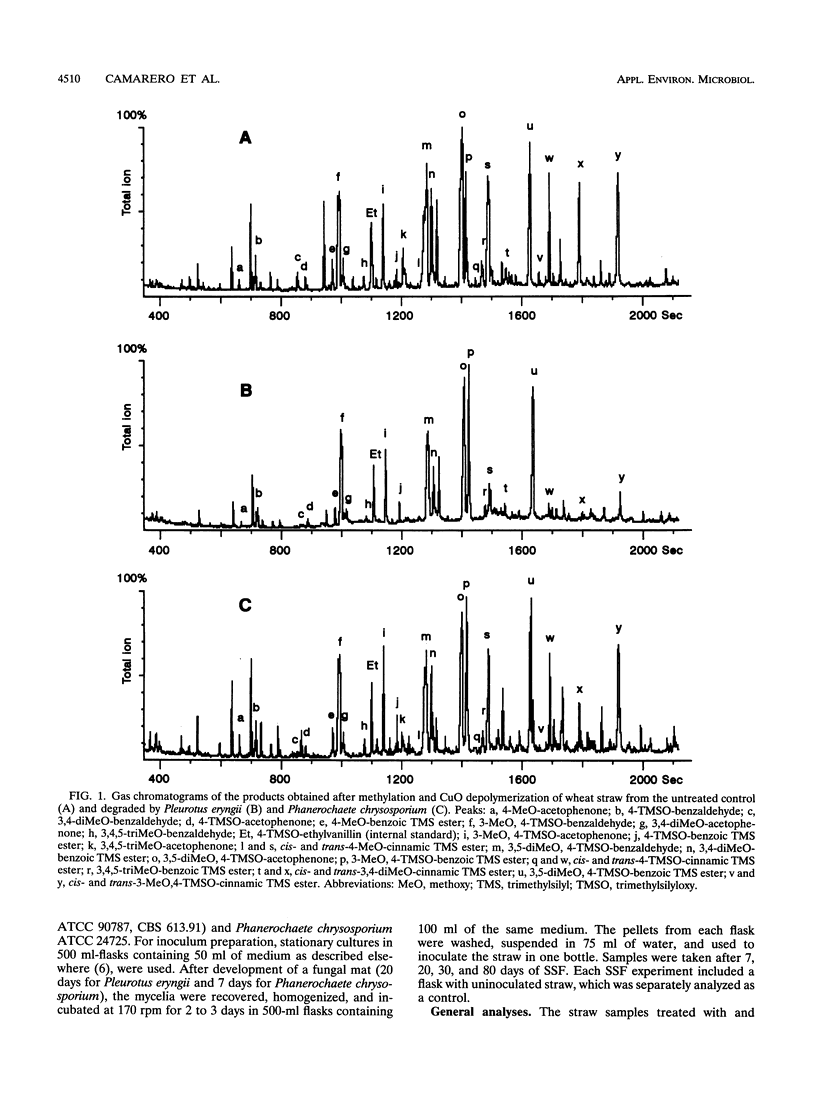
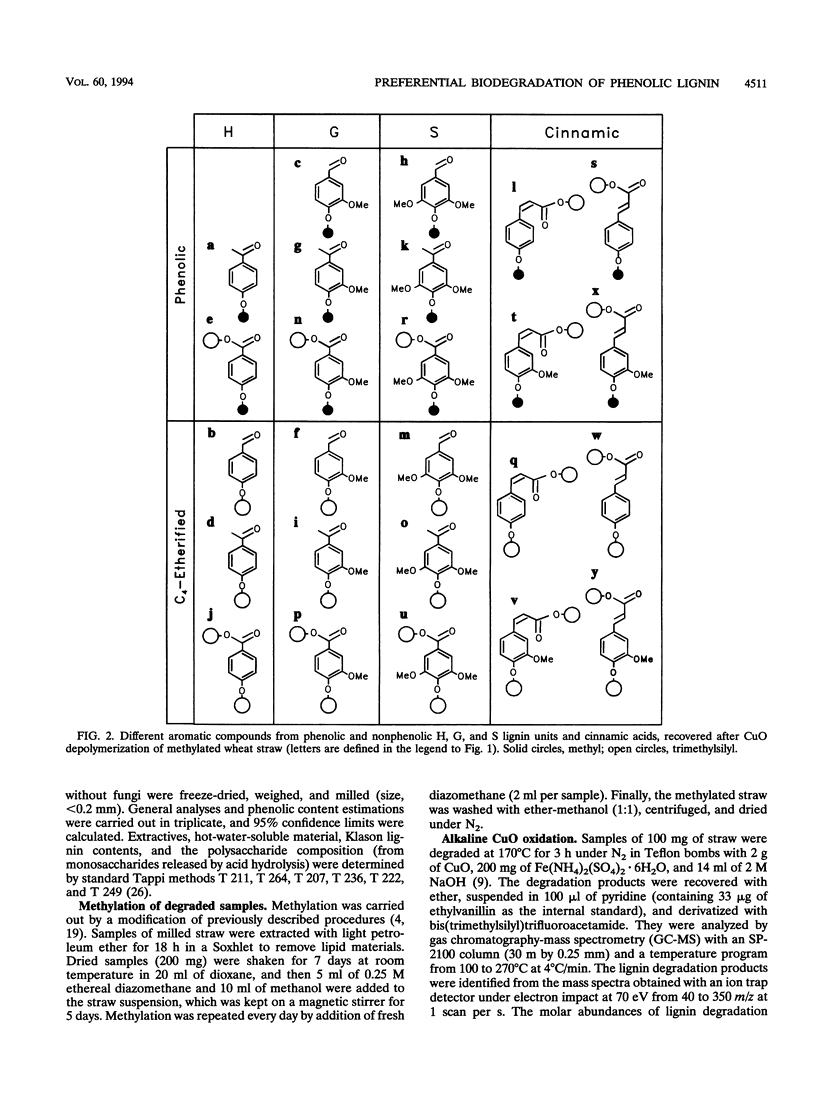
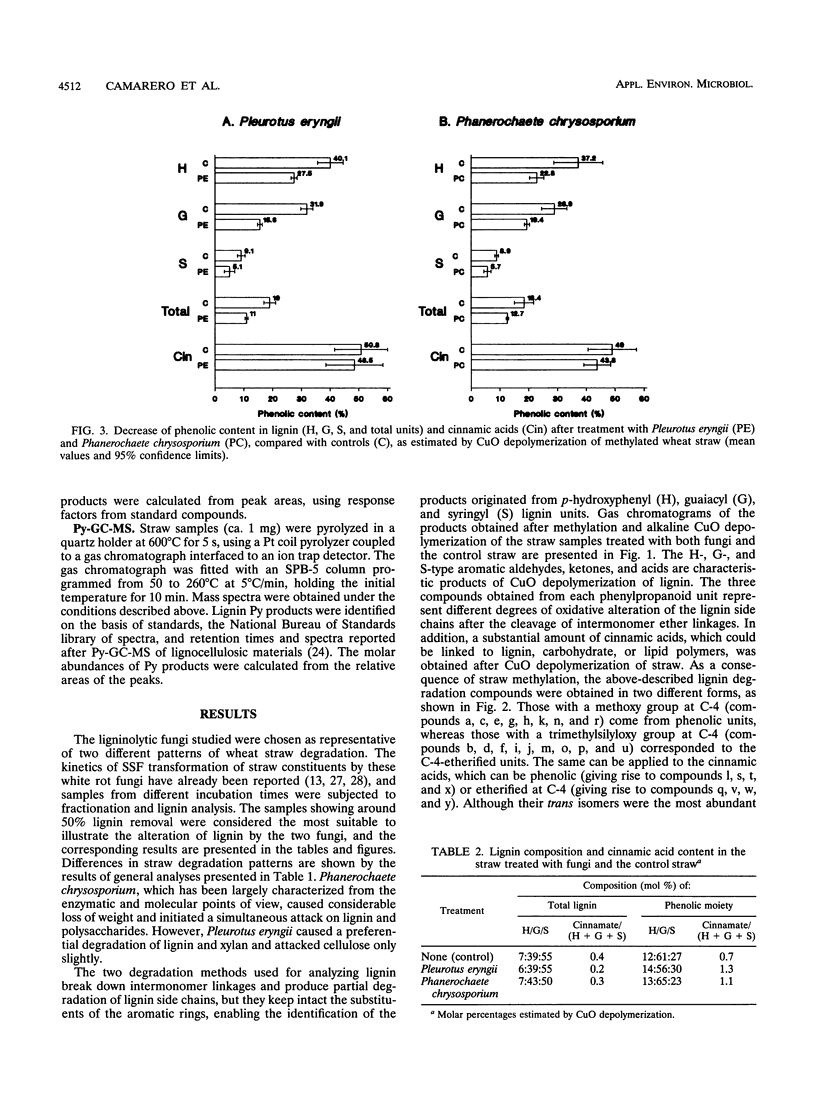
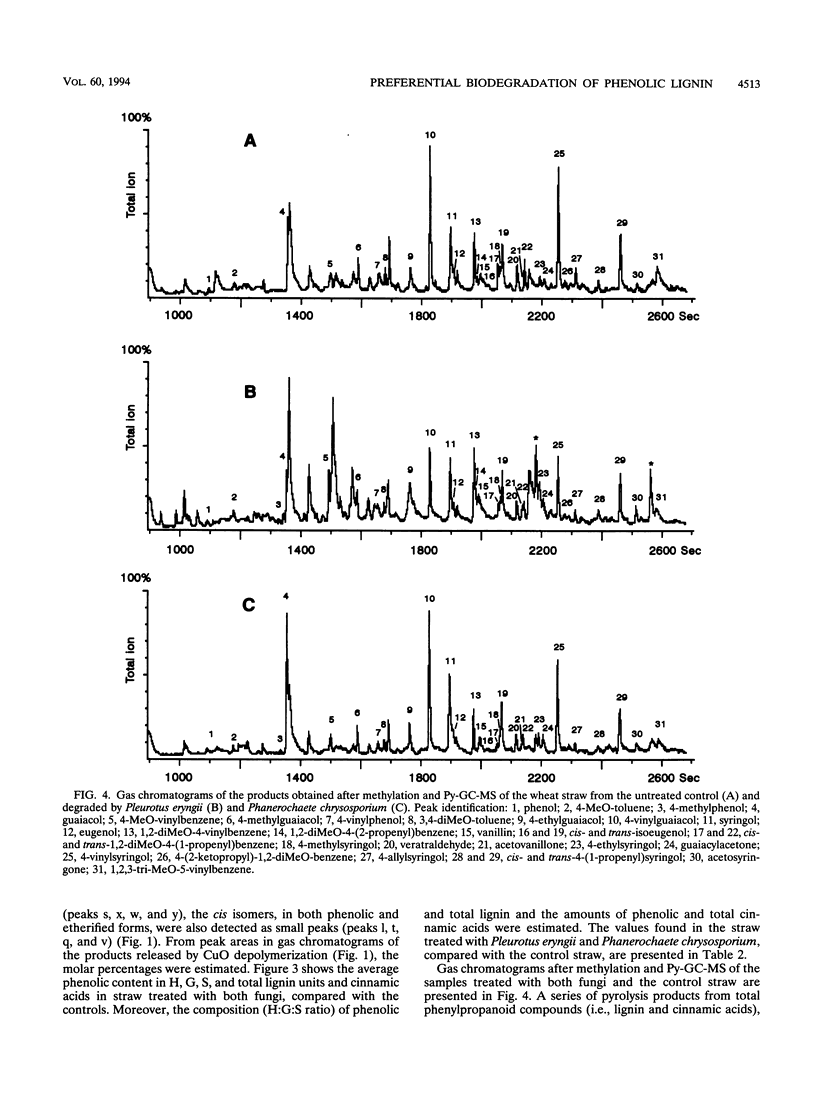
The differential biodegradation of phenolic and nonphenolic (C-4-etherified) lignin units in wheat straw treated with the white rot fungi Pleurotus eryngii and Phanerochaete chrysosporium was investigated under solid-state fermentation conditions. Two analytical techniques applied to permethylated straw were used for this purpose, i.e., alkaline CuO degradation and analytical pyrolysis (both followed by gas chromatography-mass spectrometry for product identification). Despite differences in the enzymatic machinery produced, both ligninolytic fungi caused a significant decrease in the relative amount of phenolic lignin units during the degradation process. Nevertheless, no differences in the biodegradation rates of phenolic and etherified cinnamic acids were observed. Changes in lignin composition and cinnamic acid content were also analyzed in the phenolic and nonphenolic lignin moieties. The results obtained are discussed in the context of the enzymatic mechanisms of lignin biodegradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourbonnais R., Paice M. G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990 Jul 2;267(1):99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- Guillén F., Martínez A. T., Martínez M. J. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur J Biochem. 1992 Oct 15;209(2):603–611. doi: 10.1111/j.1432-1033.1992.tb17326.x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez A., Caramelo L., Prieto A., Martínez M. J., Martínez A. T. Anisaldehyde production and aryl-alcohol oxidase and dehydrogenase activities in ligninolytic fungi of the genus Pleurotus. Appl Environ Microbiol. 1994 Jun;60(6):1783–1788. doi: 10.1128/aem.60.6.1783-1788.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J. Glyoxal oxidase of Phanerochaete chrysosporium: its characterization and activation by lignin peroxidase. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2936–2940. doi: 10.1073/pnas.87.8.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Germain G., Lapierre S., Tessier D. Performance characteristics of two bioassays and high-performance liquid chromatography for determination of flucytosine in serum. Antimicrob Agents Chemother. 1989 Aug;33(8):1403–1405. doi: 10.1128/aac.33.8.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]


