Abstract
The skeletal muscle L-type Ca2+ channel is a complex of five subunits that is specifically localized in the triad. Its primary function is the rapid activation of Ca2+ release from cytoplasmic stores in a process called excitation-contraction coupling. To study the role of α1S–β1a interactions in the incorporation of the functional channel complex into the triad, α1S and β1a [or a β1a-green fluorescent protein (GFP) fusion protein] were expressed alone and in combination in myotubes of the dysgenic cell line GLT. βGFP expressed in dysgenic myotubes that lack the skeletal muscle α1S subunit was diffusely distributed in the cytoplasm. On coexpression with the α1S subunit βGFP distribution became clustered and colocalized with α1S immunofluorescence. Based on the colocalization of βGFP and α1S with the ryanodine receptor the clusters were identified as T-tubule/sarcoplasmic reticulum junctions. Expression of α1S with and without β1a restored Ca2+ currents and depolarization-induced Ca2+ release. The translocation of βGFP from the cytoplasm into the junctions failed when βGFP was coexpressed with α1S mutants in which the β interaction domain had been altered (α1S-Y366S) or deleted (α1S-Δ351–380). Although α1S-Y366S did not associate with βGFP it was incorporated into the junctions, and it restored Ca2+ currents and depolarization-induced Ca2+ release. Thus, β1a requires the association with the β interaction domain in the I–II cytoplasmic loop of α1S for its own incorporation into triad junctions, but stable α1S–β1a association is not necessary for the targeting of α1S into the triads or for its normal function in Ca2+ conductance and excitation-contraction coupling.
The skeletal muscle dihydropyridine (DHP) receptor is a L-type Ca2+ channel that functions primarily in the fast activation of Ca2+ release from cytoplasmic stores in a process called excitation–contraction (EC) coupling (1). It is specifically localized in the triad, a junction between the transverse tubules (T-tubules) and the sarcoplasmic reticulum (SR) (2, 3). However, the mechanisms involved in the targeting and organization of the Ca2+ channels in the triad are still elusive (4). The incorporation of the DHP receptor into the junctional T-tubule membrane and the organization of the Ca2+ release channel, also called the ryanodine (RY) receptor, in the SR occur independently of each other (5–7). Thus, targeting and incorporation of DHP and RY receptors into the skeletal muscle triad appear to be intrinsic properties of these channels.
The skeletal muscle DHP receptor consists of five subunits (8). The α1S subunit forms the ion channel and contains the drug binding sites and the molecular domains for interactions with accessory channel subunits and the RY receptor. The α2δ subunit complex is a disulfide-linked heterodimer that is anchored in the T-tubule membrane by a transmembrane segment in the δ subunit. The β1a subunit is a peripheral membrane protein that associates with the DHP receptor complex via binding to a conserved motif of nine amino acids in the cytoplasmic loop between repeats I and II of the α1 subunit (9, 10). The γ subunit is a transmembrane protein that is specific for the skeletal muscle DHP receptor complex. Coexpression of various combinations of α1 subunits and accessory subunits in heterologous expression systems suggested a role of the α2δ and the β subunits in the insertion of the functional Ca2+ channel into the plasma membrane (11–13). Thus, these two subunits may also be involved in the targeting and organization of the DHP receptor in the skeletal muscle triad. However, in dysgenic myotubes, which lack the skeletal muscle α1 subunit (α1S), the α2 subunit was mistargeted (14). Thus, the α2δ subunit needs α1S for its own incorporation into the triad and therefore is an unlikely candidate for directing the DHP receptor complex into the junctional T-tubules. The β subunit is essential for the development of functional skeletal muscle. Mice with a targeted deletion of the β1a gene are paralyzed (15). β-null myotubes not only lack the β1a subunit but also show a severely reduced expression of the α1S subunit (16). Reconstitution of β-null myotubes by transient transfection with β1a restores Ca2+ currents and EC coupling (17). Thus, β1a is important for the functional expression of the α1S subunit in the skeletal muscle.
Here we used coexpression of normal and mutated skeletal muscle α1S subunits with a fusion protein of β1a and the green fluorescent protein (βGFP) in dysgenic myotubes to show that β1a needs the intact β interaction domain in the I–II cytoplasmic loop of the α1S subunit for its own incorporation into the triad. However, formation of a stable α1S/β1a complex was neither required for the targeting of α1S into the triad nor for the restoration of Ca2+ currents and EC coupling in dysgenic muscle cells.
METHODS
Transfections.
Myotubes of the homozygous dysgenic (mdg/mdg) cell line GLT were cultured as described in Powell et al. (6). At the height of myoblast fusion (2–3 days after addition of differentiation medium) GLT cultures were transfected by using a liposomal transfection reagent (DOTAP, Boehringer Mannheim). In cotransfections two or more expression plasmids were combined at equimolar concentrations to a total DNA concentration of 10 μg. This resulted in coexpression of any α1S construct and βGFP in approximately 80% of βGFP-transfected cells. One day after transfection the medium was changed, and the cultures were incubated for 2–4 days at 30°C. For expression plasmids, the coding sequence of the rabbit α1S-cDNA (18) was inserted into the plasmid pcDNA3 (Invitrogen) by digestion with HindIII. The following mutations were introduced: α1S-Y366S, a substitution of tyrosine in position 366 to serine; α1S-Δ351–380, α1S with a deletion of 30 residues in positions 351–380; and α1S-Δ351–368, with a deletion of 18 residues in positions 351–368. A fusion protein of β1a (19) with the green fluorescent protein (GFP) (20) linked to its C terminus was constructed in multiple cloning steps. For details of cloning strategies, mutation primers, and functional analysis of the constructs in a heterologous expression system see ref. 21.
GFP and Immunofluorescence Labeling.
Differentiated GLT cultures were fixed in 4% paraformaldehyde and immunostained as previously described (5) by using the monoclonal antibody 1A against the DHP receptor α1S subunit (20) and the affinity-purified antibody 5 against the type 1 RY receptor (5). For double labeling with GFP, Texas red-conjugated antibodies were used to exclude bleed-through between the red and the green channels. The antibodies were carefully characterized for their use in immunofluorescence experiments in previous studies (3, 5, 23). Controls (for example, the omission of primary antibodies and incubation with inappropriate antibodies) were routinely performed.
Patch-Clamp Recording.
Whole cell patch-clamp recordings (24) were performed with an Axopatch 200A amplifier controlled by pClamp 6.0 software (Axon Instruments, Foster City, CA). The bath solution contained (in mM): 10 CaCl2, 120 tetraethylammonium methanesulfonate, and 10 Hepes (adjusted to pH = 7.4 with tetraethylammonium-OH). Patch pipettes were filled with (in mM): 130 Cs-aspartate, 10 Hepes, 2 Mg-ATP, 2 Cs-EGTA; and 0.5 MgCl2 (adjusted to pH 7.4 with CsOH). Resistances were between 4 and 7 MΩ. Capacitative currents were compensated by using the built-in analog circuits (series resistance error was corrected for 80%). Seal resistance in the cell-attached mode was normally larger than 5 GΩ. Leak currents were subtracted by a P/4 prepulse protocol. Recordings were low pass Bessel-filtered at 1 kHz and sampled at 0.5 kHz. To test fluorescent cells (βGFP or GFP transfected) for expression of high-voltage-activated currents a voltage ramp protocol was used (from −80 mV to +80 mV over a period of 1 s). The detection threshold for high voltage-activated currents was about 10–30 pA depending on individual recording conditions. I/V curves were determined either with 1-s steps from a holding potential of −80 mV to test potentials of −40, −30… to +80 mV (+P/4 leak subtraction protocol) or from a holding potential of −40 to test potentials of −30, −20… to +80 mV (−P/4 leak subtraction protocol). Using a holding potential of −40 mV inactivated endogenous, low voltage-activated, fast inactivating Ca2+ currents, presumably of the T-type. Because there was no significant difference in the characteristics of the I/V curves obtained with either protocol, all data were pooled.
Ca2+ Recording.
Fluorescence recordings of intracellular free Ca2+ concentrations were performed as described in Flucher et al. (5) on a Zeiss Axiovert epifluorescence microscope equipped with a Photon Technology International (PTI, South Brunswick, NJ) photometer system. For use with the fluorescent Ca2+ indicators, myotubes were transfected with α1S constructs and β1a (19) instead of βGFP to avoid interference of the GFP fluorescence with the fluo-3 signal. Cultures were incubated with fluo-3 AM (5 μM) plus 0.1% Pluronic F-127 (Molecular Probes) in Hepes- and bicarbonate-buffered DMEM without phenol red for 45 min at room temperature. The fluorescence signal from a single myotube was recorded at a sampling rate of 200 Hz with PTI Oscar software. Traces were normalized by calculating the ΔF/F ratio. Electrical field stimulation was performed with a 2-ms pulse of 30 V passed across the 19-mm incubation chamber. Bath solutions contained (in mM): 145 NaCl, 5.3 KCl, 2 MgCl2, 10 Hepes, 30 glucose, pH adjusted to 7.2 with KOH, plus 2 CaCl2 in the Ca2+-containing solution only.
RESULTS
β1a Requires α1S for Its Targeting to the Triad.
GLT cells are of the homozygous dysgenic (mdg/mdg) genotype. They form myotubes that lack the α1 subunit of the skeletal muscle DHP receptor and consequently are deficient in EC coupling. Nevertheless, GLT myotubes develop T-tubule/SR junctions with normally incorporated RY receptors, and they are capable of caffeine-induced Ca2+ release (6). Transfection of GLT myotubes with an expression plasmid encoding βGFP resulted in a diffuse distribution of βGFP fluorescence (Fig. 1a). When βGFP was coexpressed with the α1 subunit of the skeletal muscle DHP receptor (α1S), the distribution of βGFP changed dramatically compared with that seen with βGFP alone. In many myotubes βGFP was no longer diffusely distributed but became concentrated in clusters throughout the myotubes (Fig. 1b). In a quantitative analysis we never found a clustered distribution of βGFP when expressed alone, whereas 45.7% of α1S/βGFP-cotransfected myotubes had clearly identifiable βGFP clusters (Table 1). Cotransfected myotubes in which βGFP did not accumulate in clusters included those myotubes that only expressed the βGFP but not the α1S plasmid, in which case the βGFP distribution was diffuse. The remainder most likely represents myotubes in which T-tubule/SR junctions have not yet developed, and therefore α1S itself was not clustered (see below). As expected, this population of myotubes was larger in cultures with an overall poor differentiation. The distinct distribution patterns—diffuse for βGFP alone, clustered for βGFP plus α1S—were confirmed by immunofluorescence labeling with a specific antibody for the β subunit (not shown). Double labeling experiments of cultures transfected with βGFP and α1S showed that βGFP clusters were colocalized with clusters of the α1S subunit and with RY receptor clusters (see below). Because these two channels are localized in the T-tubules (or the plasma membrane) and in the terminal SR cisternae, respectively, their colocalization is indicative of a junctional localization. Thus, βGFP requires the coexpression of α1S for its incorporation into T-tubule/SR (or plasma membrane/SR) junctions.
Figure 1.
Clustering of β1a in dysgenic myotubes depends on coexpression of α1S with intact β interaction domain. βGFP is expressed alone (a) or in combination with either the wild-type α1S (b), the α1S-Y366S (c), or the α1S-Δ351–380 mutant (d). The β1a subunit forms clusters only when coexpressed with the wild-type α1S subunit (b). When coexpressed with the mutated α1S subunits the distribution of βGFP is diffuse, as if expressed alone (compare c and d with a). N, myotube nuclei. (Bar, 20 μm.)
Table 1.
Subcellular distribution of α1S and βGFP in transfected dysgenic myotubes
| Protein | Detected by | Myotube numbers and percentages (in parentheses) for individual constructs
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| βGFP
|
βGFP + α1S
|
βGFP + α1S-Y366S
|
βGFP + α1S-Δ351–380
|
||||||
| Clustered | Diffuse | Clustered | Diffuse | Clustered | Diffuse | Clustered | Diffuse | ||
| βGFP | GFP | 0 (0.0) | 208 (81.6) | 102 (45.7) | 73 (32.7) | 1 (0.7) | 121 (88.3) | 0 (0.0) | 91 (90.1) |
| α1S | Anti-α1S | 223 (63.9) | 106 (30.4) | 73 (44.2) | 72 (43.6) | 0 (0.0) | 137 (86.7) | ||
“Diffuse” for βGFP means cytoplasmic diffuse; for α1S, diffuse in ER/SR.
Triad Targeting of β1a Depends on the Intact β Interaction Domain in α1S.
To examine whether specific interactions between the α1S and the β1a subunits are necessary for the incorporation of βGFP into the T-tubule/SR junctions, we constructed α1S expression plasmids in which the β interaction domain in the I–II cytoplasmic loop has been deleted or mutated (21). In the point mutation (α1S-Y366S) the tyrosine in position 366, which is conserved in all α1 isoforms, has been substituted with serine. The α1S-Δ351–380 deletion comprises the entire β interaction domain, and α1S-Δ351–368 comprises the N-terminal two-thirds of the β interaction domain. Because both deletion mutants gave identical results throughout this study, data of only one, α1S-Δ351–380, will be shown. Mutations in corresponding regions of α1A have been shown to eliminate the β-binding capacity of α1A (9). Coexpression of βGFP with the wild-type and the mutant α1S constructs resulted in different βGFP distribution patterns. Whereas coexpression with the wild-type α1S caused clustering of βGFP (Fig. 1b), clustering was not observed with the mutated α1S subunits (Fig. 1 c and d). Cotransfection of βGFP with the α1S-Y366S or either one of the deletions resulted in diffuse βGFP distribution, similar to the pattern observed when βGFP was expressed alone (Fig. 1a). Only one of 137 myotubes cotransfected with βGFP and α1S-Y366S had a cluster-like distribution of βGFP, and none cotransfected with α1S-Δ351–380 (Table 1). Thus, mutations in the β interaction domain of the I–II cytoplasmic loop of α1S disrupt the incorporation of the β1a subunit into T-tubule/SR junctions.
Triad Targeting of α1S Does Not Require Stable Association with β1a.
Double labeling of cotransfected GLT myotubes with βGFP and a specific antibody against the α1S subunit of the DHP receptor showed that all examined α1S constructs were expressed but that βGFP colocalized only with the wild-type α1S subunit (Fig. 2). Thus, the mutations in the β interaction domain specifically interfere with the ability of the two subunits to form a stable complex and not with the biosynthesis and expression of the α1S subunit. Interestingly, however, the different mutant α1S constructs showed differential distribution patterns among themselves. Whereas wild-type α1S and α1S-Y366S achieved a normal clustered distribution in many myotubes (Fig. 2 b and d), the deletion mutants did not form clusters (Fig. 2f). Even though α1S-Y366S did not lead to clustering of βGFP, α1S-Y366S itself was clustered (compare Fig. 2c with 2d). Apparently, stable association of βGFP is not required for triad targeting of α1S-Y366S. In contrast, α1S-Δ351–380 was not clustered but localized in a reticular/tubular membrane system (Fig. 2f) that was densest in the perinuclear region. The labeling pattern closely resembled that previously observed with Ca2+-ATPase immunolabeling in young myotubes (25), suggesting that the cytoplasmic structure containing α1S-Δ351–380 represents the endo-sarcoplasmic reticulum. Deletion of the β interaction domain in α1S may have perturbed both binding to the β subunit and the export of the channel from the biosynthesis organelle. Quantitative comparison showed that 63.9% of myotubes transfected with wild-type α1S and 44.2% with α1S-Y366S, but none of the α1S-Δ351–380-transfected myotubes achieved a clustered distribution of the α1S subunit (Table 1).
Figure 2.
Differential distribution of the β1a subunit and α1S subunit mutants in cotransfected dysgenic myotubes. βGFP is visualized with GFP fluorescence (upper row) and α1S subunits by immunofluorescence (lower row). a and b, wild-type α1S is colocalized with βGFP in clusters (examples indicated with arrows). c and d, the α1S-Y366S mutant is localized in clusters, but βGFP is diffusely distributed throughout the myotube. e and f, the α1S-Δ351–380 mutant is localized in a dense reticular membrane system and βGFP is diffuse. The insets show a flat region of a myotube at twice the magnification. Note the tubular nature of the membrane structure containing α1S-Δ351–380 and the clearly distinct diffuse distribution of βGFP. (Bar, 20 μm.)
Similar results were obtained when GLT myotubes were transfected with α1S constructs plus β1a instead of βGFP. Transfections with wild-type or α1S-Y366S alone, without βGFP or β1a, also resulted in clustered α1S distribution (not shown), possibly because of the action of the endogenous β1a in dysgenic myotubes. Thus, the type of β1a subunit (endogenous β1a, exogenous β1a, or βGFP) had no influence on the distribution of the α1S constructs. Double immunolabeling of transfected GLT myotubes with antibodies against the DHP receptor α1S subunit and the RY receptor showed that both wild-type α1S and α1S-Y366S were colocalized with the RY receptor (Fig. 3 a–d). But even though RY receptor clusters occurred at normal frequencies in α1S-Δ351–380-transfected myotubes, α1S-Δ351–380 remained localized in the endosarcoplasmic reticulum (Fig. 3 e and f). Thus, the incorporation of α1S-Y366S into T-tubule/SR junctions can occur without the stable association of the β1a subunit. However, the deletion of the β interaction domain leads to failure of α1S-Δ351–380 targeting into the junctions.
Figure 3.
Incorporation of α1S wild type and α1S-Y366S mutant into T-tubule/SR junctions. Double immunolabeling of transfected dysgenic myotubes with antibodies against the α1S subunit (upper row) and against the RY receptor (lower row) is shown. Colocalization of the wild-type α1S (a and b) or the α1S-Y366S mutant (c and d) with the RY receptor in clusters is indicative of a junctional location of the two channels (examples indicated with arrows). e and f, incorporation of α1S-Δ351–380 into junctions fails, even though the RY receptor forms normal clusters in these myotubes. Note that the RY clusters are also formed in myotubes not expressing α1S (asterisk). N, myotube nuclei. (Bar, 20 μm.)
Calcium Currents and EC Coupling Do Not Depend on Stable Association of α1S and β1a.
The wild-type and the Y366S mutant of the DHP receptor α1S subunit differ in only a single residue; both get inserted into the T-tubule/SR junctions, but only the wild-type α1S forms a stable association with the β1a subunit. Thus, we analyzed whether this difference affects the functions of the DHP receptor as L-type Ca2+ channel and as voltage sensor in EC coupling.
For patch-clamp analysis of Ca2+ currents wild-type or mutant α1S constructs were expressed in GLT myotubes with and without β1a plus the GFP marker protein. Expression of high-voltage-activated Ca2+ currents was tested by using a ramp protocol for depolarization. GLT myotubes transfected with α1S or α1S-Y366S without β1a or βGFP expressed detectable Ca2+ currents in 69% (α1S) and in 29% (α1S-Y366S) of GFP-positive cells (Table 2). Cotransfection with β1a (or βGFP) resulted in a slight increase of the fraction of current expressing cells with α1S and in a significant (P < 0.05) increase to 67% (69% for βGFP) in cells transfected with α1S-Y366S. Thus, expression of functional α1S-Y366S channels, which do not form stable complexes with β1a, is enhanced when the cytoplasmic concentration of β1a is increased by the expression of exogenous β1a. In contrast, deletion of the β interaction domain severely impaired the expression of Ca2+ currents. Only one in 11 myotubes transfected with α1S-Δ351–368 and none with α1S-Δ351–380 expressed a detectable current. Therefore the function of the deletion mutants could not be further analyzed. Table 2 shows that coexpression of βGFP or β1a plus GFP with wild-type or point mutant α1S gave similar results, indicating that the GFP fusion protein had the same properties as β1a. However, for reasons of better comparison with the β− condition, in which GFP had to be used separately, the combination of the two plasmids and not the fusion protein was used for analysis of current properties. I/V curves of myotubes expressing the wild-type α1S and of myotubes expressing α1S-Y366S show that peak current densities were significantly increased (P < 0.05) at all voltages when the cultures were cotransfected with β1a (Fig. 4). This is further evidence that α1S-Y366S is sensitive to coexpression of β1a even though it does not form a stable complex with β1a.
Table 2.
Expression frequency of high-voltage-activated Ca2+ currents in transfected GTL cells
| Coexpressed with | Percentage of positive cells (total no. of cells tested)
|
|||
|---|---|---|---|---|
| α1S | α1S-Y366S | α1S-Δ351–368 | α1S-Δ351–380 | |
| GFP | 69 (36) | 29 (73) | — | — |
| β1a/GFP | 81 (27) | 67 (42) | — | — |
| βGFP | 89 (89) | 69 (59) | 9 (11) | 0 (15) |
Figure 4.
Modulation of Ca2+ currents by β1a in dysgenic myotubes expressing the wild-type or Y366S mutant α1S subunit. Patch-clamp recordings of myotubes transfected with α1S (a) or α1S-Y366S (b) with (β+) and without (β−) β1a plus GFP as an expression marker are shown. The left panels show representative current traces for step depolarizations from −40 mV to +20 mV and +40 mV (asterisks). The right panels show the average I/V curves for the peak current densities (n, number of analyzed myotubes; when the current did not peak during the 1-s pulse the current value at the end of the pulse was used). In both myotubes expressing α1S and myotubes expressing α1S-Y366S, the peak current density is significantly (P < 0.05) increased when β1a is coexpressed.
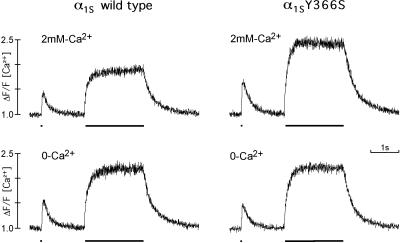
Voltage-dependent gating of the DHP receptor rapidly activates the opening of the Ca2+ release channel (RY receptor) in the SR. In skeletal muscle this signal transduction mechanism is independent of the slowly activating L-type Ca2+ current. We recorded changes in the concentration of free cytoplasmic Ca2+ ([Ca2+]) in transfected GLT myotubes loaded with the fluorescent Ca2+ indicator fluo-3 AM. Field depolarization with 2-ms current pulses across the culture chamber gave rise to [Ca2+] transients in myotubes transfected with either wild-type α1S or α1S-Y366S, both with β1a (Fig. 5). For both α1S constructs the kinetics of the [Ca2+] transients were similar and resembled those of depolarization-induced [Ca2+] transients recorded in immature normal skeletal myotubes (23). [Ca2+] transients in response to a single stimulating current pulse were small compared with the [Ca2+] reached during tetanic stimulation at 20 Hz for 2 s. This indicates that during a single twitch only a fraction of the total releasable Ca2+ was released from the SR. Removal of Ca2+ from the bath solution did not stop the [Ca2+] transients in myotubes transfected with either one of the α1S constructs (Fig. 5, lower row). This demonstrates that the transient increase of [Ca2+] did not arise from influx of Ca2+ through the L-type Ca2+ channel and that Ca2+ release from the SR was not mediated by Ca2+ influx via a mechanism termed Ca2+-induced Ca2+ release. Thus, the observed [Ca2+] transients have the characteristics of skeletal muscle-type depolarization-induced Ca2+ release. The mutation in the β interaction domain of α1S-Y366S and the resulting failure to stably associate with β1a had no apparent effect on its ability to function as voltage sensor in skeletal muscle EC coupling.
Figure 5.
Depolarization-induced Ca2+ transients in dysgenic myotubes expressing the wild-type or Y366S mutant α1S subunit. Transfected cultures were loaded with the fluorescent Ca2+ indicator fluo-3 AM and depolarized by passing a 2-ms, 30-V pulse (dot underneath traces) and a 2-s train of pulses at 20 Hz (bar underneath traces) across the culture chamber. Both, α1S- and α1S-Y366S-transfected myotubes responded with a transient increase in cytoplasmic free [Ca2+] to individual and tetanic stimulation. After changing to a bath solution not containing Ca2+, the same myotubes still responded with a [Ca2+] transient to depolarization (lower traces). Ca2+-sensitive fluorescent signals were normalized to baseline values (ΔF/F); duration of trace was 6 s.
DISCUSSION
Incorporation of β1a into Developing Triads.
The diffuse distribution of βGFP when expressed in dysgenic myotubes suggests a cytoplasmic localization of the β subunit in myotubes lacking the α1S subunit of the DHP receptor. βGFP is excluded from the nuclei and does not show an association with the plasma membrane. Unlike other β isoforms (12, 13), β1a in skeletal muscle is apparently not anchored to membranes. Most importantly, βGFP was never observed in a clustered distribution in the absence of the α1S subunit, even though RY receptor immunolabeling showed that GLT myotubes developed RY clusters at high frequency. On coexpression with α1S, however, βGFP formed clusters. Based on the colocalization of the junctional T-tubule proteins (the DHP receptor subunits) with a junctional SR protein (the RY receptor), these clusters are identified as T-tubule/SR junctions (or in some cases plasma membrane/SR junctions). Thus, βGFP requires the α1S subunit for its incorporation into the T-tubule/SR junctions.
The distinct diffuse or clustered βGFP distribution patterns were confirmed with β antibody staining that colocalized with the βGFP fluorescence. The βGFP fusion protein is incorporated into the T-tubule/SR junctions despite the GFP attached to its C terminus and despite the presence of endogenous β subunit in dysgenic myotubes. Moreover, in functional analyses βGFP effects were identical with those obtained with wild-type β1a (see also ref. 21). Thus, βGFP can substitute for and compete with the wild-type β subunit, and expression of βGFP in addition to the endogenously expressed β subunit appears to be a valid method to determine the distribution of the β subunit in skeletal myotubes. The present results differ from those of Gregg and colleagues (15) who reported cases of clustered β immunolabeling in dysgenic primary myotubes. But colocalization with RY receptor clusters to identify these clusters unambiguously as T-tubule/SR junctions was not shown. In our hands, no clustered β distribution was observed in nontransfected GLT myotubes, even when the myotubes showed a high degree of RY receptor clustering. Alterations of the β interaction domain either by deletion or by a single tyrosine-to-serine substitution caused the failure of βGFP clustering in cotransfected dysgenic myotubes. Thus, it is the specific interaction of the β1a subunit with the conserved β interaction domain of the cytoplasmic loop connecting repeats I and II of the α1S subunit that anchors the β subunit in the junction. This mode of α1S–β1a interaction excludes the possibility that β1a itself plays an essential role in anchoring the α1S subunit in the junctions.
Effects of β1a on Targeting and Function of α1S.
Are α1S–β1a interactions required for the incorporation of functional α1S into the T-tubule/SR junction? Two mutant α1S constructs that both failed to form a stable complex with β1a, as seen with βGFP and immunofluorescence labeling, revealed different targeting properties. Whereas deletion of the β interaction domain caused the failure of α1S-Δ351–380 targeting, the point mutation in the β interaction domain did not prevent the incorporation of α1S-Y366S into the triad. The deletion mutant was retained in a reticular cytoplasmic membrane system, presumably the endo-sarcoplasmic reticulum. One possible explanation is that deletion of the β interaction domain blocks interactions with β1a that in turn causes failure of export from the endoplasmic reticulum or failed transport to the T-tubule/SR junctions. These results suggest that interactions between α1S and β1a are necessary for their incorporation into the triads. This is consistent with data from β knock-out mice, which showed a severely reduced expression of the α1 subunit as seen with immunocytochemistry and patch-clamp recording of Ca2+ currents (15, 16). Expression of β1a in β-null myotubes restored Ca2+ currents and EC coupling (17). Consequently, β1a may play an important role in the translocation or incorporation of α1S into the triad, and either the targeted deletion of β or the impediment of α1S–β1a interactions results in a failure of α1S incorporation into the T-tubule/SR junctions.
However, our results obtained with the Y366S mutant of α1S make it necessary to distinguish between α1S/β1a complex formation and α1S–β1a interactions. Despite the fact that α1S-Y366S does not show stable association with β1a it is incorporated into the triad, fulfills its normal function in EC coupling, and most importantly, it is still sensitive to coexpression of β1a in electrophysiological analysis. When β1a was coexpressed with α1S-Y366S, current expression and the peak current densities were increased. Thus α1S-Y366S and β1a can interact without forming a stable complex. This observation is consistent with the original report on the β interaction domain (9). Whereas binding of β1b to the Y392S mutant of the α1A subunit (which corresponds to the skeletal Y366S used in the present study) was abolished, Ca2+ currents through the Y392S mutant were merely reduced by 9-fold compared with the wild type. Thus, the tyrosine-to-serine substitution may lower the affinity for β1a binding, preventing the α1S/β1a complex formation but not functional interaction. The decreased affinity may partially have been compensated by the expression of exogenous β1a, leading to increased current densities.
Depolarization-induced Ca2+ release was also restored to an equal extent by transfection with α1S and α1S-Y366S. This indicates that both α1S constructs, which became normally incorporated into the T-tubule/SR junctions, could also function in skeletal muscle-type EC coupling. This was, however, not dependent on the stable association with the β1a subunit. Studies on β-null myotubes have shown that the β subunit of the DHP receptor is essential for the development of a contractile skeletal muscle. However, it remained unclear whether this was because of the deficiency in α1S expression induced by the targeted disruption of the β gene or because of the lack of β1a-induced modulation of the α1S subunit. Our present data add the finding that, whereas α1S–β1a interactions are important, the stable association of α1S and β1a in the triad is not necessary for targeting and function of the α1S subunit in skeletal myotubes.
A Dual Nature of α1S–β1a Interactions in Skeletal Muscle.
In normal muscle the β1a subunit appears to bind tightly to the β interaction domain of the α1S subunit. The two proteins form a stable complex that may facilitate the incorporation of the channel into the triad and its function in EC coupling (see model in Fig. 6). Inhibition of α1S–β1a interactions by deletion of the β interaction domain in the I–II cytoplasmic loop of α1S indicates the importance of this domain by blocking incorporation of the α1 subunit into the triad and function. A more subtle alteration of the β interaction domain reveals that stable association of α1S and β1a is not required for functional interaction of the two subunits. The free β1a subunit may reversibly interact with α1S-Y366S and thus fulfill its role in the targeting of the channel to the triad and perhaps in the functional modulation of α1S in Ca2+ conduction and EC coupling.
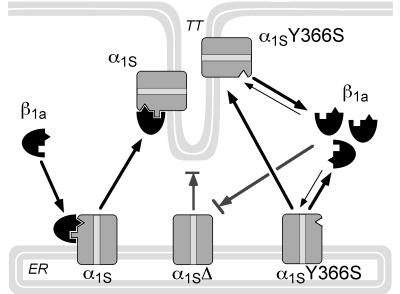
Figure 6.
Model of α1S–β1a interactions in the assembly of the DHP receptor complex in the skeletal muscle triad. β1a without the α1S subunit is localized in the cytoplasm. On coexpression β1a and α1S form a complex in the endoplasmic reticulum that is then incorporated into the junctional T-tubules (TT). Deletion of the β interaction domain in the I–II cytoplasmic loop blocks β1a association and export of the α1S mutant (α1S-Δ) from the endoplasmic reticulum. A point mutation (α1S-Y366S) in the high affinity β1a binding site of α1S (symbolized by the rectangular nipple) inhibits α1S/β1a complex formation but not the functional incorporation of the mutated α1S-Y366S into the T-tubules. Dynamic interactions between free cytoplasmic β1a and a low affinity interaction site (symbolized by the triangular notch) may modulate Ca2+ currents of α1S-Y366S and may be involved in the translocation of α1S-Y366S into the T-tubules.
Acknowledgments
We thank Drs. S. Fleischer, S. Froehner, and J. Striessnig for generous gifts of antibodies and Dr. H. Hoflacher for excellent technical assistance. This work was supported in part by grants from the Fonds zur Förderung der wissenschaftlichen Forschung, Austria (S06612-MED), the Austrian National Bank (3535), and the European Commissions Training and Mobility of Researchers Network (ERBFMRXCT 960032) (to B.E.F.). B.E.F. is an APART (Austrian Programme for Advanced Research and Technology) Fellow of the Austrian Academy of Sciences.
ABBREVIATIONS
- DHP
dihydropyridine
- EC
excitation–contraction
- T-tubules
transverse tubules
- SR
sarcoplasmic reticulum
- βGFP
β1a–green fluorescent protein fusion protein
- RY
ryanodine
References
- 1.Rios E, Ma J, Gonzales A. J Muscle Res Cell Motil. 1991;12:127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen A O, Shen A C-Y, Arnold W, Leung A T, Campbell K P. J Cell Biol. 1989;109:135–147. doi: 10.1083/jcb.109.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flucher B E, Morton M E, Froehner S C, Daniels M P. Neuron. 1990;5:339–351. doi: 10.1016/0896-6273(90)90170-k. [DOI] [PubMed] [Google Scholar]
- 4.Flucher B E, Franzini-Armstrong C. Proc Natl Acad Sci USA. 1996;93:8101–8106. doi: 10.1073/pnas.93.15.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flucher B E, Andrews S B, Fleischer S, Marks A R, Caswell A H, Powell J A. J Cell Biol. 1993;123:1161–1174. doi: 10.1083/jcb.123.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell J A, Petherbridge L, Flucher B E. J Cell Biol. 1996;134:375–387. doi: 10.1083/jcb.134.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Protasi F, Franzini-Armstrong C, Allan P D. Biophys J. 1997;72:13. (abstr.) [Google Scholar]
- 8.Catterall W A. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 9.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch T P, Campbell K P. Nature (London) 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 10.DeWaard M, Scott V E S, Pragnell M, Campbell K P. FEBS Lett. 1996;380:272–276. doi: 10.1016/0014-5793(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 11.Isom L L, DeJongh K S, Catterall W A. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 12.Chien A J, Zhao X, Shirokov R E, Puri T S, Chang C F, Sun D, Rios E, Hosey M M. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 13.Brice N L, Berrow N S, Campbell V, Page K M, Brickley K, Tedder I, Dolphin A C. Eur J Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 14.Flucher B E, Phillips J L, Powell J A. J Cell Biol. 1991;115:1345–1356. doi: 10.1083/jcb.115.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregg R G, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell J A, Coronado R, Powers P A. Proc Natl Acad Sci USA. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strube C, Beurg M, Powers P A, Gregg R G, Coronado R. Biophys J. 1996;71:2531–2543. doi: 10.1016/S0006-3495(96)79446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beurg M, Sukhareva M, Strube C, Powers P A, Gregg R G, Coronado R. Biophys J. 1997;73:807–818. doi: 10.1016/S0006-3495(97)78113-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanabe T, Beam K G, Powell J A, Numa S. Nature (London) 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 19.Mitterdorfer J, Froschmayr M, Grabner M, Striessnig J, Glossmann H. FEBS Lett. 1994;352:141–145. doi: 10.1016/0014-5793(94)00938-4. [DOI] [PubMed] [Google Scholar]
- 20.Heim R, Cubitt A B, Tsien R Y. Nature (London) 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 21.Neuhuber, B., Gerster, U., Mitterdorfer, J., Glossmann, H. & Flucher, B. E. (1998) J. Biol. Chem. 273, in press. [DOI] [PubMed]
- 22.Morton M E, Froehner S C. J Cell Biol. 1987;262:11904–11907. [PubMed] [Google Scholar]
- 23.Flucher B E, Andrews S B, Daniels M P. Mol Biol Cell. 1994;5:1105–1118. doi: 10.1091/mbc.5.10.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 25.Flucher B E. Dev Biol. 1992;154:245–260. doi: 10.1016/0012-1606(92)90065-o. [DOI] [PubMed] [Google Scholar]