Abstract
The aim of the present work was to characterize the odontoblastic proliferation, differentiation and matrix mineralization in culture of the recently established M2H4 rat cell line. Proliferation was assessed by cell counts, differentiation by RT-PCR analysis and mineralization by alizarin red staining, atomic absorption spectrometry and FTIR micro-spectroscopy. The results showed that M2H4 cell behavior closely mimics in vivo odontoblast differentiation, with in particular, temporally regulated expression of DMP-1 and DSPP. Moreover, the mineral phase formed by M2H4 cells was similar to that in dentin from rat incisors. Finally, since it was reported that in mice, TGF-β1 over-expression in vivo leads to an hypomineralization similar to that observed in dentinogenesis imperfecta type II, effects of TGF-β1 on mineralization in M2H4 cell culture were studied. Treatment with TGF-β1 dramatically reduced mineralization whereas positive control treatment with BMP-4 enhanced it, suggesting that M2H4 cell line is a promising tool to explore the mineralization mechanisms in physio-pathologic conditions.
Keywords: Animals; Biological Markers; metabolism; Bone Morphogenetic Proteins; pharmacology; Calcification, Physiologic; drug effects; physiology; Calcium; analysis; metabolism; Cell Differentiation; Cell Proliferation; Cells, Cultured; Dentin; drug effects; metabolism; Extracellular Matrix Proteins; genetics; metabolism; Female; Gene Expression; Odontoblasts; cytology; drug effects; metabolism; Phosphoproteins; genetics; metabolism; Protein Precursors; genetics; metabolism; RNA, Messenger; metabolism; Rats; Rats, Wistar; Reverse Transcriptase Polymerase Chain Reaction; Spectroscopy, Fourier Transform Infrared; Transforming Growth Factor beta1; pharmacology
INTRODUCTION
Dentin is a mineralized tissue whose composition and mode of formation are relatively similar to those of bone. The dentin extracellular matrix (ECM) is composed of approximately 47 % (v/v) mineral, 30 % (v/v) protein and 21 % (v/v) water (1). In dentin and bone, type I collagen (COLL-1) accounts for about 90% of the protein fraction and most of the dentin non-collagenous proteins (NCP) are also expressed in bone. However, some NCP appear to be more specifically expressed in dentin even though they are also found, albeit weaker, in other tissues. For instance, odontoblasts express dentin matrix protein-1 (DMP-1) and dentin sialophosphoprotein (DSPP), the latter giving rise to dentin sialoprotein (DSP) and dentin phosphoprotein (DPP) after proteolytic cleavage (2–4). If the function of DSP remains poorly understood, it seems likely that DPP is involved in matrix mineralization. It is secreted from the odontoblast process directly at the mineralization front (5) and has a high affinity for calcium ions (6) and hydroxyapatite crystals (7). In addition, DPP may bind collagen fibrils by covalent (8,9) or electrostatic bonds (10) to regulate either or both mineralization (11,12) and fibrillogenesis (13,14). Defective expression of DPP is suspected to be the cause of dentinogenesis imperfecta type II (DI-II) (15). Interestingly, DI-II is characterized by a decreased mineral density (16), defects in collagen/crystal association (17,18) and increased collagen fibril diameter and orientation (17). Several studies have investigated the possible role of DPP on in vitro models of crystal formation and collagen fibrillogenesis (9,11–13,19,20). However, since these models can hardly reproduce the process of cell-mediated dentin mineralization, in vitro models of mineralizing odontoblasts appear important.
Today, if some cell lines were reported to express DSPP and mineralize their ECM (21,22), there has been no detailed analysis of the mineral phase formed in culture and there is still great uncertainty whether the mineralization is similar to the in vivo crystal formation. In this context, the aim of the present study was to further characterize the recently developed M2H4 rat cell line to propose a new tool to decipher the implication of DSPP in physiological and pathological dentinogenesis. The M2H4 cell line was developed from the RPC-C2A pulp cell line (23). Whereas the parent RPC-C2A cell line does not express DPP (24) nor mineralizes the ECM (23), M2H4 cells were selected by their ability to form crystals in culture, and they were found to express DSPP transcripts (23). This cell line could thus be a useful tool to study odontoblast differentiation and dentin ECM mineralization in vitro. However, little or no information is available concerning M2H4 proliferation, differentiation pattern, and the nature of the mineral phase formed in culture. In this context, we aimed at investigating in more details the behavior of this cell line. Moreover, since TGF-β1 over-expression in mouse dentin in vivo was shown to induce dental disorders similar to those found in DI-II (25), we investigated ECM mineralization in response to TGF-β1, as compared to mineralization in response to an “osteogenic” factor of the TGF-β family, bone morphogenetic protein-4 (BMP-4). This work is the first step of an effort to provide new insights into the molecular events implicated in dentinogenesis both in physiological and pathological conditions.
MATERIALS AND METHODS
Cell culture plasticware was purchased from Falcon (Becton-Dickinson, Franklin Lakes, NJ) and Corning-Costar (D. Dutscher, Brumath, France). Fetal calf serum (PCS) was obtained from D. Dutscher. MEM, α-MEM, glutamine, antibiotics, trypsin/ethylene-diamine tetraacetic acid (EDTA), bovine serum albumin (BSA) were obtained from Life Technologies Ltd. (Paisley, UK). Transforming growth factor-β1 (TGF-β1) was obtained from R&D Systems (Abingdon, UK) and BMP-4 was generously provided by Genetics Institute (Cambridge, UK). TGF-β1 and BMP-4 were respectively dissolved as concentrated solutions in 4M hydrochlorid acid/BSA (HCl/BSA) and 0.1% BSA in phosphate buffered saline (PBS/BSA). Trizol reagent, DNase I and Taq DNA polymerase were obtained from Life Technologies. Avian myeloblastosis virus-reverse transcriptase (AMV-RT), random hexamers and recombinant ribonuclease inhibitor were purchased from Promega (Madison, WI). All other chemicals were from standard laboratory suppliers and were of the highest purity available.
Rat incisors were extracted from three week-old Wistar females. All teeth were fixed in 10% formaldehyde (pH 7.4). Before investigation by Fourier transform infrared micro-spectroscopy (FTIR-M), teeth were dehydrated through increasing ethanol gradients and embedded in glycolmethylmethacrylate (GMA), as described elsewhere (26).
Cell and culture conditions
As previously described (23), M2H4 cells were routinely grown in a maintenance medium consisting in MEM containing 10% ECS, 1% antibiotics and 1% glutamine. Cells were subcultured once a week using trypsin/EDTA, and maintained at 37 °C in a humidified atmosphere of 5% CO2 in air. To induce odontoblast differentiation, MEM was switched to α-MEM. To induce ECM mineralization, 3 mM inorganic phosphate (Pi) were added to the culture medium on day 8. Pi was added as a mixture of NaH2PO4 and Na2HPO4 (pH 7.3). Cells were treated with 10 ng/ml TGF-β1, 100 ng/ml BMP-4, or vehicles from day 6 to day 21 and medium was replaced every two days.
RNA isolation
M2H4 Cells, with a final density of 10,000 cells/cm2, were seeded in 25 cm2 flasks for RNA isolation. After indicated times, media were removed, cell layers rinsed with RNase free PBS and stored at −80°C. Total RNA was extracted using the Trizol ® reagent according to the manufacturer’s instructions. Briefly, lysis of the cells in Trizol was followed by centrifugation at 10,000 g at 4°C for 15 minutes in the presence of chloroform. The upper aqueous phase was collected and the RNA was precipitated by addition of isopropanol and centrifugation at 7,500 g at 4°C for 5 minutes. RNA pellets were washed with cold 75% ethanol, dried, reconstituted with sterile water, and quantified by spectrometry.
Reverse transcription and polymerase chain reaction (RT-PCR) analysis
After DNase I digestion, RNA samples (2 μg) were reverse-transcribed using AMV-RT and random hexamers in a total volume of 30 μl. Template cDNAs (2.5 μl) were then amplified in a typical 25 μl PCR reaction containing 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1 μM of the respective primers, 200 μM dNTP and 2.5 units of Taq DNA polymerase. The magnesium chloride concentration was 1.5 mM. The absence of DNA contamination in RNA preparations was tested by including RNA samples that had not been reverse-transcribed. Primer sequences and annealing temperatures are detailed in table 1 (27–29). Amplifications were carried out in an Eppendorf master cycler (VWR, Brumath, France) under the following conditions: denaturation for 3 minutes at 94°C followed by cycles of 30 s denaturation at 94° C, 30 s annealing at the primer specific temperature and 45 s elongation at 72°C. All PCR results show amplification products obtained in the linear range of amplification. Analysis of RNA levels after normalization to GAPDH (glyceraldehyde phosphate dehydrogenase) levels was realized by densitometry (Leica Q500, Cambridge, UK).
Table 1.
primers used for RT-PCR analysis(sequences, annealing temperature (T), size of PCR products (bp), and reference, GAPDH: glyceraldehyde phosphate dehydrogenase, ALP: alkaline phosphatase, DMP-1: dentin matrix protein 1, DSPP: dentin sialophosphoprotein).
| Name | Forward (F) and Reverse (R) sequence | T annealing | bp | reference |
|---|---|---|---|---|
| GAPDH | F 5′-ACC ACA GTC CAT GCC ATC AC-3′
R 5′-TCC ACC ACC CTG TTG CTG TA-3′ |
62°C | 452 | (27) |
| COLL-1 | F 5 ′-CCA ATC TGG TTC CCT CCC AC-3′
R 5 ′-TGG TAA GOT TGA ATG CAC TT-3′ |
62°C | 181 | (28) |
| ALP | F 5′-CTT CTT GCT AGT GGA AGG-3′
R 5 ′-CCT GOT AGT TGT TGT GAG-3′ |
51°C | 346 | unpublished |
| DMP-1 | F 5′-GAA GAC TGT TAT CCT TAG G-3′
R 5′-GTG ATC CCC TTT AGA TTC C-3′ |
62°C | 672 | (27) |
| DSPP | F 5 ′-CAC ATC CAG GAA CCG CAG CAC-3′
R 5′CCT TAG TCT CCT TTG CCT C-3′ |
60°C | 330 | (29) |
Fourier transform infrared micro-spectroscopy
Sections (2 μm-thick) of GMA-embedded rat teeth were obtained with a Supercut 2050 microtome (Reichert-Jung, Heidelberg, Germany). After removal of the culture medium, cell layers from M2H4 culture (6 multiwell plates) were collected with a scalpel. Tooth sections and cell layers were placed on a barium fluoride (BaF2) disk for FTIR microscopic investigation. Spectra were recorded with a Magna-IR 550 spectrometer (Nicolet, Trappes, France) equipped with an IR-plan Advantage microscope (Spectra-Tech, Shelton, CT; X15 Reflachromat lens) fitted with a high-sensitivity mercury cadmium tellurite (MCT) detector. Sample positioning was obtained with a motorized x-y stage under computer control. FTIR data were acquired with the spatial resolution provided by a 20 × 20 μm2 aperture in order to prevent diffraction artifacts and maximize the signal/noise ratio. Infrared spectra were recorded at 4 cm−1 resolution with either 512 or 1024 interferograms co-added and Happ-Genzel apodization. Omnic software (Nicolet, Trappes, France) was used for data analysis. Residual H2O and CO2 absorptions were automatically subtracted, and GMA peaks were subtracted when necessary. Before mineral analysis by deconvolution of the ν1ν3PO4 domain (k=2.3 and σ=22.5 cm−1), collagen absorption was subtracted, especially to eliminate weak contributions at 1080 and 1030 cm−1 interfering with phosphate absorption (30). The ν1ν3PO4 domain was analyzed because its underlying bands provide information on apatite crystallinity (31). Whereas the bands at 1030 cm−1 and 1090 cm−1 are found in well-crystallized apatites, those at 1020, 1110 or 1125 cm−1 are typical of poorly organized crystals.
Analytical methods
To obtain data on M2H4 cell proliferation, cells were plated in 6 multiwell plates at a density of 10,000 cells/cm2 and cell number was measured by counting trypan blue stained cells under a contrast-phase microscope. Calcium deposition in cell layers was firstly investigated with Alizarin Red staining. Briefly, cells in 6 multiwell plates were stained with 1 ml per well 2% Alizarin Red at pH 4.2 for 2 minutes. ECM mineralization was then quantified as the amount (μg) of total calcium (Ca) in cell layers (12 multiwell plates), as determined by atomic absorption spectrometry at 422.7 nm (Unicam 989 AA spectrometer, SOLAR), after extraction of the cells with 4 M HCl containing 1 % LaCl3. Protein content (mg) was determined with the Pierce Coomassie Plus assay reagent (Pierce, Rockford, IL). Each experiment was repeated at least once with similar results. Results are expressed as mean ± SEM of triplicate determinations. Comparative studies of means were performed using one way analysis of variance followed by a post-hoc test (Fisher’s projected least significant difference) with a statistical significance at p<0.05.
RESULTS
In order to complete the characterization of M2H4 cell behavior, we investigated firstly cell proliferation in culture conditions developed by Ritchie et al. (23). Cell counting after Trypan Blue staining indicated that M2H4 cell proliferation may be maximal between days 7 and 14 and may slow thereafter (Fig. 1).
Fig. 1. M2H4 cell proliferation.
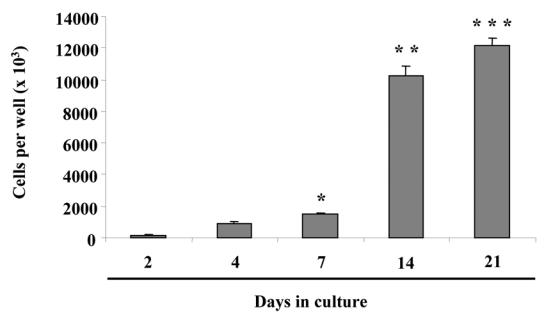
Cells were cultured for the indicated times in conditions described in the “materials and methods” section. Cell proliferation was assessed by counting after Trypan Blue staining. *p<0.05 compared with cell number on day 2. **p<0.05 compared with cell number on day 7 and ***p<0.05 compared with cell number on day 14.
Then we investigated expression of odontoblastic markers at the mRNA level by RT-PCR between days 7 and 20 (Fig. 2). We studied in particular the expression of type I collagen, which in dentin accounts for approximately 90% of the total protein fraction, and alkaline phosphatase (ALP), which by generating Pi from phosphorylated compounds is supposed to play an important role during the first steps of mineralization. In addition, we explored the expression pattern at the mRNA level of two major dentin NCP, DSPP and DMP-1, whose temporally regulated expression is a distinctive characteristic of odontoblasts. Densitometric analysis of bands in agarose gels revealed that M2H4 expression of COLL-1 and ALP was nearly constant from day 7 to day 20, whereas expression of DMP-1 and DSPP appeared to be regulated during differentiation. DMP-1 appeared as an early marker of M2H4 differentiation because its expression increased until day 11 and was relatively constant thereafter. Conversely, DSPP was rather a late differentiation marker, being weakly expressed until day 11 and increasing between day 11 and day 20.
Fig. 2. Time course of odontoblast marker expression during M2H4 cell differentiation.
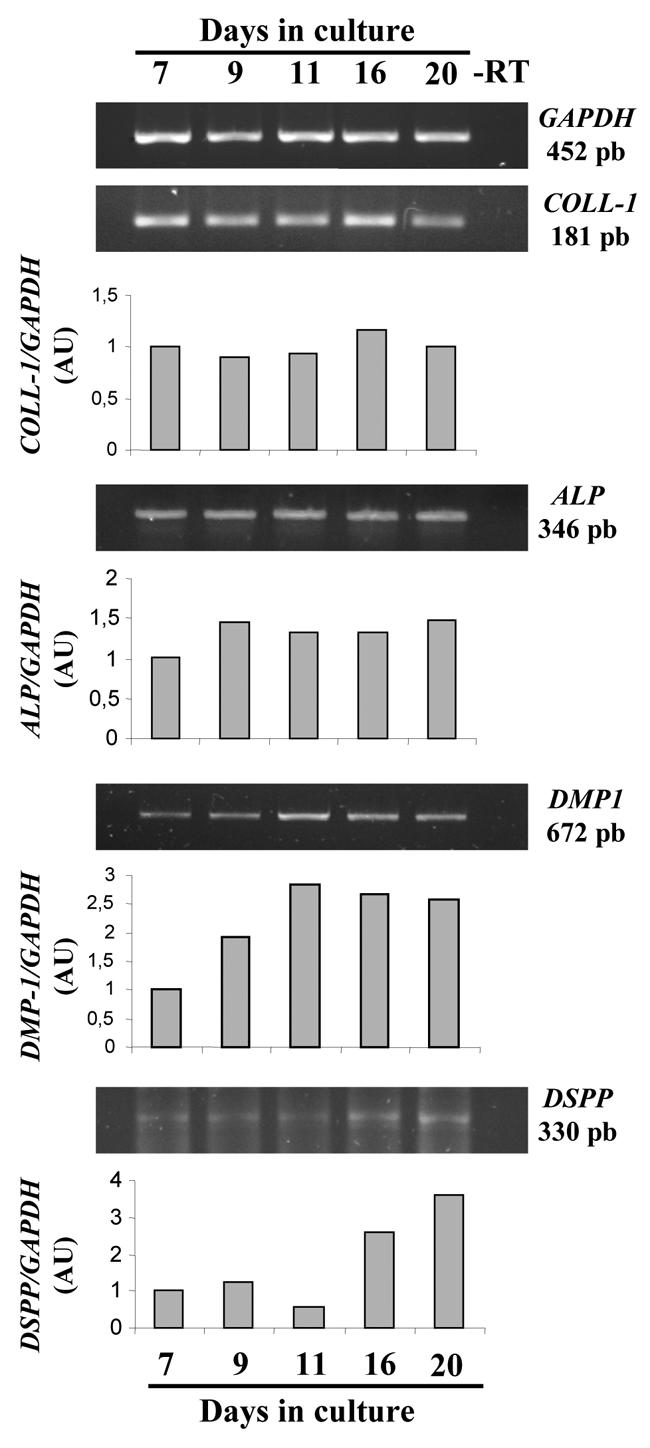
Cells were cultured for the indicated times in conditions described in the “materials and methods” section. Representative agarose gels of RT-PCR analysis (−RT: control without reverse transcriptase) and densitometric analysis after normalisation to GAPDH levels (ALP: alkaline phosphatase, AU: arbitrary units, COLL-I: type I collagen, GAPDH: glyceraldehyde phosphate dehydrogenase).
In the present study, we aimed at determining whether the M2H4 cell line is suitable for the study of ECM mineralization. Therefore, we investigated quantitatively and qualitatively the mineralization in M2H4 culture. As illustrated by alizarin red staining at day 28 (Fig. 3A), M2H4 cells cultured in medium supplemented from day 8 with 3 mM Pi accumulate calcium in cell layers whereas cells grown without Pi supplementation appear much less stained. Quantification of calcium content in cell layers was then performed by atomic absorption spectrometry. In control conditions (1 mM Pi), M2H4 cells failed to mineralize the ECM until 20 days culture. In contrast, when the culture medium was supplemented with 3 mM Pi on day 8, mineralization was induced from day 11, and increased dramatically until day 20 (Fig. 3B).
Fig. 3. Quantitative and qualitative analysis of the mineral phase deposited in M2H4 cell culture.
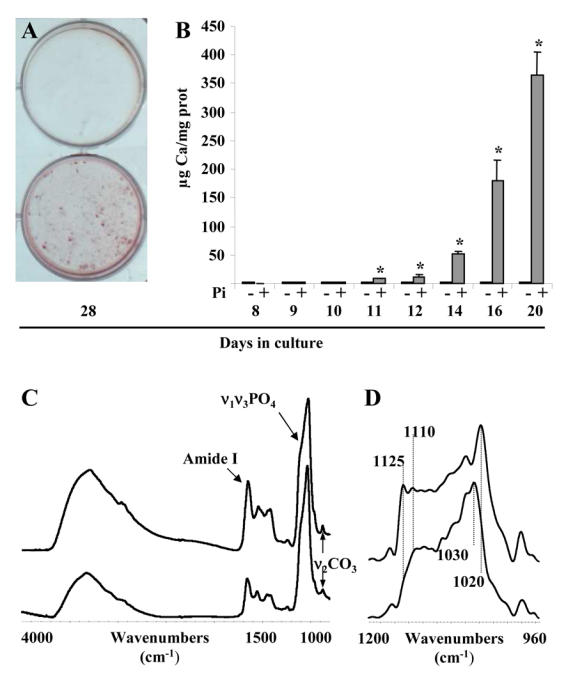
Cells were cultured for the indicated times in conditions described in the “materials and methods” section. (A) Alizarin red staining (upper well: without Pi supplementation; lower well: with Pi supplementation; individual representations of a representative triplicate experiment). (B) Calcium deposition and protein content in cell layers as determined respectively by atomic absorption spectrometry and spectrometry after Coomassie blue staining. (C) Typical FTIR-M spectra of M2H4 cell layer after 21 days culture (upper spectrum) and a mature dentin region from a three week-old rat (lower spectrum). (D) Deconvolution of the ν1ν3PCX domain of M2H4 cell layer after 21 days culture (upper spectrum) and of a mature dentin region from a three week-old rat (lower spectrum). *p<0.05 compared with control without Pi supplementation.
Crystal composition and organization were then investigated by FTIR microspectroscopy on cell layers cultured for 21 days and were compared with those in mature regions of dentin sections from three week old rats. Firstly, spectra suggest that the mineral/protein ratio was weaker in cell layers after 21 days culture than in mature rat dentin, because the protein Amide I band appeared much weaker with respect to ν1ν3PO4 domain in spectra of rat dentin (Fig. 3C). Deconvolution of the ν1ν3PO4 domain revealed that crystals in culture consisted in a carbonated apatite similar to that in rat dentin slices (Fig. 3D). However, the high intensities in the culture spectrum of the bands at 1020, 1110 and 1125 cm−1 indicated that crystals in culture were less crystallized than those in fully mineralized rat dentin. The weaker mineral/protein ratio and crystallinity suggest that ECM matrix was not fully mineralized on day 21.
Finally, because it has been reported that TGF-β1 overexpression in vivo induces dentin disorders similar to those observed in DI-II (25), we investigated the effects of TGF-β1 on mineralization in M2H4 cell culture (Fig. 4A). As a positive control, we studied the effects of BMP-4 (Fig. 4B), which is an “osteogenic” member of the TGF-β super-family. TGF-β1 and BMP-4 were added to the culture medium just before the onset of mineralization and calcium deposition was quantified after 10 days treatment. The results showed that whereas calcium deposition was significantly stimulated by BMP-4, it was dramatically inhibited by TGF-β1. The content of the inorganic phase remaining after TGF-β1 treatment was even so reduced that it could not be analyzed by FTIR microspectroscopy.
Fig. 4. effects of TGF-β and BMP-4 on calcium deposition in M2H4 cell culture.
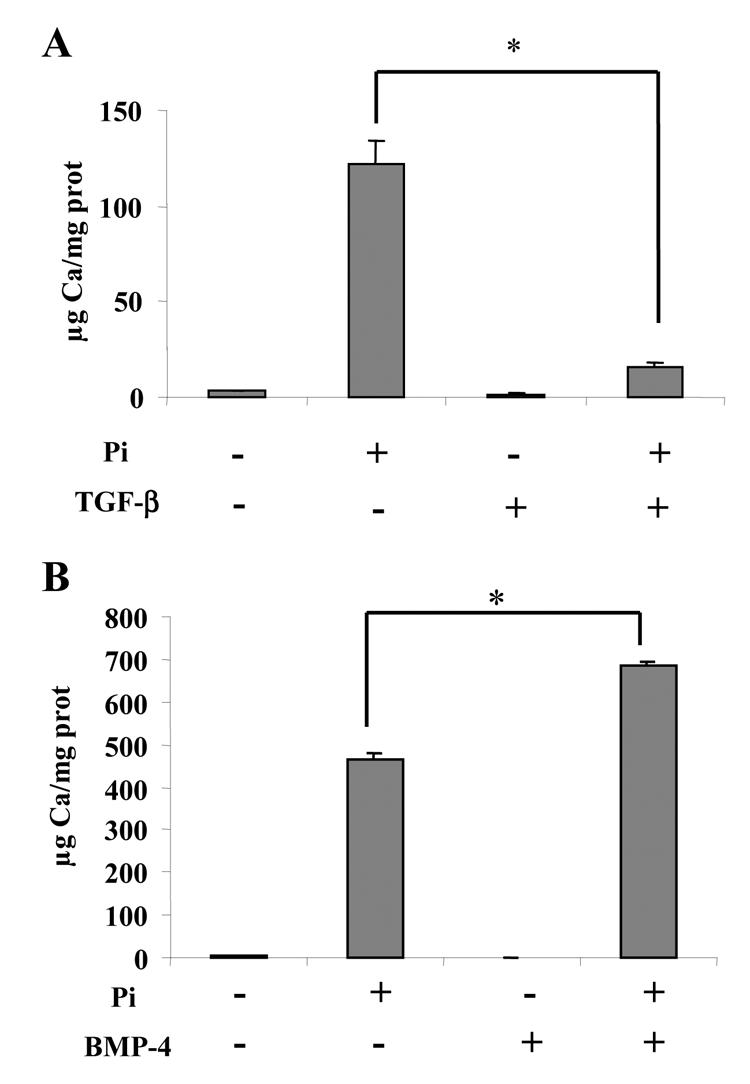
Cells were treated on day 6 with 10 ng/ml TGF-β, 100 ng/ml BMP-4 or vehicles. On day 21, calcium deposition and protein content in cell layers were determined respectively by atomic absorption spectrometry and spectrometry after Coomassie blue staining, as detailed in the “materials and methods” section. *p<0.05 compared with control conditions (treatment with respective vehicle).
DISCUSSION
Because odontoblasts are post-mitotic cells that are difficult to maintain in culture for a long time due to their finite life span, several cell lines have recently been developed from mouse (32,33), and rat (22,34) cells. In the present investigation, we analyzed the behavior of the new odontoblast M2H4 cell line, developed from the RPC-C2A rat pulp cells, which in presence of ascorbic acid, expresses COLL-1 and DSPP transcripts, and has the capacity to mineralize the extracellular matrix (23). Since in culture conditions developed by Ritchie et al, mineralization begins on day 11, we analyzed proliferation, expression of odontoblast differentiation markers and mineralization for 20 days. We investigated expression at the mRNA level of type I collagen that represents nearly 90% of odontoblast synthesized proteins, and ALP that is highly expressed by cells undergoing mineralization process. Moreover, we explored the expression of DMP-1 and DSPP, which may not be specifically expressed by odontoblasts (35–37), but are considered as the main hallmarks of odontoblasts. Our results indicated that differentiation of M2H4 cells closely mimics odontoblast differentiation in vivo (35,38). COLL-1 and ALP were expressed early in the M2H4 differentiation and their expression remained relatively constant during M2H4 maturation (38). The constant expression of ALP agrees with ALP activity, which was also nearly constant during the same period (data not shown). It has been shown that ALP expression may decrease when the mineralization process is initiated (27). In the present study, we have investigated ALP expression until day 20, it is therefore possible that ALP levels may decrease later when mineralization is more advanced. DMP-1 expression increased before the beginning of mineralization and was relatively constant thereafter (22,35). In contrast, the increase in DSPP expression seemed to occur after the M2H4 proliferation phase and coincided more or less with the time course of mineralization. This temporal expression pattern is consistent with odontoblast differentiation during in vivo dentinogenesis (35,38). Finally, it was shown that over-expression of DMP-1 in RPC-C2A cells induced the expression of DSPP (39). Since in our study, DMP-1 expression was up-regulated before the increase in DSPP expression, it is not totally unconceivable that DSPP expression in RPC-C2A-derived M2H4 cells is also under the control of DMP-1.
Whereas in recent studies reporting the development of other odontoblast cell lines, the mineral phase formed in culture was not qualitatively analyzed, the inorganic phase deposited by M2H4 cells was studied by FTIR-M, and compared with crystals from rat dentin. Indeed, because one of the main functions of odontoblasts is to mineralize the ECM, a convenient odontoblast cell line must form crystals identical to those formed in vivo. In the previous report of the M2H4 cell line, it was shown that crystals were deposited in association with the extracellular matrix (23), but it remained to be determined whether crystal composition was similar to that in vivo. Our results revealed that crystals formed in vivo and in vitro after 21 days culture consist similarly in a non-stoichiometric apatite. The mineral/protein ratio was however inferior in cell layers as compared with mature rat dentin, suggesting that the ECM was not fully mineralized after 21 days. This was confirmed by the lower crystallinity of the inorganic phase formed in vitro, which likely resulted from a weaker maturation time (26). Therefore, it seems reasonable to assume that crystals deposited after 21 days in culture may be similar to the immature ones located near the mineralization front in dentin in vivo (40).
In addition, we questioned whether mineralization in M2H4 culture could be modulated by factors known to regulate either or both DSPP expression and ECM mineralization. It has been shown recently that in vivo TGF-β1 over-expression by mouse odontoblasts resulted in inhibition of DSPP expression, decreased mineralization and development of dental disorders similar to those occurring during DI-II (25). In vitro, TGF-β1 was shown to down-regulate DSPP expression in the murine MO6-G3 cell line (41). Conversely, BMP-4, another member of the TGF-β super-family expressed during tooth development (42), may stimulate odontoblast differentiation (43). In M2H4 culture, mineralization in cell layers was dramatically reduced after treatment with TGF-β1 from day 6 to day 21, suggesting that M2H4 cells express TGF-β1 receptors. In contrast, the positive control treatment with BMP-4 significantly increased mineralization, consistently with an “osteogenic” effect of this TGF-β super-family member (44). These in vitro results agree with the report indicating that TGF-β1 may decrease mineralization in vivo (25). However, the dramatic inhibition of mineralization after TGF-β1 treatment prevented precise analysis of crystal composition. Today, new strategies to regulate specifically DSPP expression by M2H4 cells, based on DSPP antisense and over-expression, is under intense investigation to provide us with further insights into the role of DSPP in the physio-pathology of dentinogenesis.
In conclusion, the M2H4 model, which reproduces in vitro the in vivo odontoblast differentiation and dentin ECM mineralization appears as a promising tool. By modulating specifically DSPP expression, this model may provide us with new information on the mineralization process in physiologic and pathologic conditions.
Acknowledgments
The authors thank R. Brion and T. Rouillon for technical assistance.
References
- 1.Legeros RZ. Calcium phosphates in enamel, dentin and bone. In: Myers HM, editor. Calcium phosphate in oral biology and medicine. Kanger; New York: 1991. p. 108. [Google Scholar]
- 2.Gu K, Chang SR, Slaven MS, Clarkson BH, Rutherford RB, Ritchie HH. Human dentin phosphophoryn nucleotide and amino acid sequence. Eur J Oral Sci. 1998;106(6):1043–1047. doi: 10.1046/j.0909-8836..t01-9-.x. [DOI] [PubMed] [Google Scholar]
- 3.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272(2):835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie HH, Wang LH. Sequence determination of an extremely acidic rat dentin phosphoprotein. J Biol Chem. 1996;271(36):21695–21698. doi: 10.1074/jbc.271.36.21695. [DOI] [PubMed] [Google Scholar]
- 5.Rahima M, Tsay TG, Andujar M, Veis A. Localization of phosphophoryn in rat incisor dentin using immunocytochemical techniques. J Histochem Cytochem. 1988;36(2):153–157. doi: 10.1177/36.2.3335773. [DOI] [PubMed] [Google Scholar]
- 6.Marsh ME. Binding of calcium and phosphate ions to dentin phosphophoryn. Biochemistry. 1989;28(1):346–352. doi: 10.1021/bi00427a047. [DOI] [PubMed] [Google Scholar]
- 7.Fujisawa R, Kuboki Y. Preferential adsorption of dentin and bone acidic proteins on the (100) face of hydroxyapatite crystals. Biochim Biophys Acta. 1991;1075(1):56–60. doi: 10.1016/0304-4165(91)90074-q. [DOI] [PubMed] [Google Scholar]
- 8.Curley-Joseph J, Veis A. The nature of covalent complexes of phosphoproteins with collagen in the bovine dentin matrix. J Dent Res. 1979;58(6):1625–1633. doi: 10.1177/00220345790580061201. [DOI] [PubMed] [Google Scholar]
- 9.Lee SL, Veis A. Studies on the structure and chemistry of dentin collagen-phosphophoryn covalent complexes. Calcif Tissue Int. 1980;31(2):123–134. doi: 10.1007/BF02407173. [DOI] [PubMed] [Google Scholar]
- 10.Traub W, Jodaikin A, Arad T, Veis A, Sabsay B. Dentin phosphophoryn binding to collagen fibrils. Matrix. 1992;12(3):197–201. doi: 10.1016/s0934-8832(11)80062-4. [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Arsenault AL, Yamauchi M, Kuboki Y, Crenshaw MA. Mineral induction by immobilized phosphoproteins. Bone. 1997;21(4):305–311. doi: 10.1016/s8756-3282(97)00149-x. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Yamauchi M, Abiko Y, Matsuda K, Crenshaw MA. In vitro apatite induction by phosphophoryn immobilized on modified collagen fibrils. J Bone Miner Res. 2000;15(8):1615–1619. doi: 10.1359/jbmr.2000.15.8.1615. [DOI] [PubMed] [Google Scholar]
- 13.Cocking-Johnson D, Sauk JJ. The interaction of bovine dentine phosphophoryn and collagen during fibrillogenesis of collagen in vitro. Biochim Biophys Acta. 1983;742(1):49–53. doi: 10.1016/0167-4838(83)90357-6. [DOI] [PubMed] [Google Scholar]
- 14.Gelman RA, Conn KM, Termine JD. The effects of phosphoproteins on collagen self-assembly in tail tendon and incision dentin from rats. Biochim Biophys Acta. 1980;630(2):220–224. doi: 10.1016/0304-4165(80)90424-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Zhao J, Li C, Gao S, Qiu C, Liu P, Wu G, Qiang B, Lo WH, Shen Y. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27(2):151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 16.Kerebel B, Daculsi G, Menanteau J, Kerebel LM. The inorganic phase in dentinogenesis imperfecta. J Dent Res. 1981;60(9):1655–1660. doi: 10.1177/00220345810600090401. [DOI] [PubMed] [Google Scholar]
- 17.Herold RC. Fine structure of tooth dentine in human dentinogenesis imperfecta. Arch Oral Biol. 1972;17(6):1009–1013. doi: 10.1016/0003-9969(72)90125-2. [DOI] [PubMed] [Google Scholar]
- 18.Kinney JH, Pople JA, Driessen CH, Breunig TM, Marshall GW, Marshall SJ. Intrafibrillar mineral may be absent in dentinogenesis imperfecta type II (DI-II) J Dent Res. 2001;80(6):1555–1559. doi: 10.1177/00220345010800061501. [DOI] [PubMed] [Google Scholar]
- 19.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Concentration-dependent effects of dentin phosphophoryn in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990;11(1):55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- 20.Termine JD, Eanes ED, Conn KM. Phosphoprotein modulation of apatite crystallization. Calcif Tissue Int. 1980;31(3):247–251. doi: 10.1007/BF02407188. [DOI] [PubMed] [Google Scholar]
- 21.Lundquist P, Ritchie HH, Moore K, Lundgren T, Linde A. Phosphate and calcium uptake by rat odontoblast-like MRPC-1 cells concomitant with mineralization. J Bone Miner Res. 2002;17(10):1801–1813. doi: 10.1359/jbmr.2002.17.10.1801. [DOI] [PubMed] [Google Scholar]
- 22.Hao J, Narayanan K, Ramachandran A, He G, Almushayt A, Evans C, George A. Odontoblast cells immortalized by telomerase produce mineralized dentin-like tissue both in vitro and in vivo. J Biol Chem. 2002;277(22):19976–19981. doi: 10.1074/jbc.M112223200. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie HH, Liu J, Kasugai S, Moller P. A mineralizing rat dental pulp cell subline expressing collagen type I and dentin sialoprotein-phosphophoryn transcripts. In Vitro Cell Dev Biol Anim. 2002;38(1):25–29. doi: 10.1290/1071-2690(2002)038<0025:AMRDPC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Yokota M, Nagata T, Ishida H, Wakano Y. Clonal dental pulp cells (RDP4-1, RPC-C2A) synthesize and secrete osteopontin (SPP1, 2ar) Biochem Biophys Res Commun. 1992;189(2):892–898. doi: 10.1016/0006-291x(92)92287-8. [DOI] [PubMed] [Google Scholar]
- 25.Thyagarajan T, Sreenath T, Cho A, Wright JT, Kulkarni AB. Reduced expression of dentin sialophosphoprotein is associated with dysplastic dentin in mice overexpressing transforming growth factor-beta 1 in teeth. J Biol Chem. 2001;276(14):11016–11020. doi: 10.1074/jbc.M010502200. [DOI] [PubMed] [Google Scholar]
- 26.Magne D, Weiss P, Bouler JM, Laboux O, Daculsi G. Study of the maturation of the organic (type I collagen) and mineral (nonstoichiometric apatite) constituents of a calcified tissue (dentin) as a function of location: a Fourier transform infrared microspectroscopic investigation. J Bone Miner Res. 2001;16(4):750–757. doi: 10.1359/jbmr.2001.16.4.750. [DOI] [PubMed] [Google Scholar]
- 27.Yokose S, Kadokura H, Tajima Y, Fujieda K, Katayama I, Matsuoka T, Katayama T. Establishment and characterization of a culture system for enzymatically released rat dental pulp cells. Calcif Tissue Int. 2000;66(2):139–144. doi: 10.1007/s002230010028. [DOI] [PubMed] [Google Scholar]
- 28.Churg A, Gilks B, Dai J. Induction of fibrogenic mediators by fine and ultrafine titanium dioxide in rat tracheal explants. Am J Physiol. 1999;277(5 Pt 1):L975–982. doi: 10.1152/ajplung.1999.277.5.L975. [DOI] [PubMed] [Google Scholar]
- 29.Dey R, Son HH, Cho MI. Isolation and partial sequencing of potentially odontoblast-specific/enriched rat cDNA clones obtained by suppression subtractive hybridization. Arch Oral Biol. 2001;46(3):249–260. doi: 10.1016/s0003-9969(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 30.Liu KZ, Dixon IM, Mantsch HH. Distribution of collagen deposition in cardiomyopathic hamster hearts determined by infrared microscopy. Cardiovasc Pathol. 1999;8(1):41–47. doi: 10.1016/s1054-8807(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 31.Rey C, Shimizu M, Collins B, Glimcher MJ. Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase of calcium phosphate in bone and enamel and their evolution with age: 2. Investigations in the nu3PO4 domain. Calcif Tissue Int. 1991;49(6):383–388. doi: 10.1007/BF02555847. [DOI] [PubMed] [Google Scholar]
- 32.Hanks CT, Fang D, Sun Z, Edwards CA, Butler WT. Dentin-specific proteins in MDPC-23 cell line. Eur J Oral Sci. 1998;106(Suppl 1):260–266. doi: 10.1111/j.1600-0722.1998.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 33.MacDougall M, Thiemann F, Ta H, Hsu P, Chen LS, Snead ML. Temperature sensitive simian virus 40 large T antigen immortalization of murine odontoblast cell cultures: establishment of clonal odontoblast cell line. Connect Tissue Res. 1995;33:1–3. 97–103. doi: 10.3109/03008209509016988. [DOI] [PubMed] [Google Scholar]
- 34.Lundgren T, Nilsson M, Ritchie HH, Linde A. Junctional proteins and Ca2+ transport in the rat odontoblast-like cell line MRPC-1. Calcif Tissue Int. 2001;68(3):192–201. doi: 10.1007/s002230010015. [DOI] [PubMed] [Google Scholar]
- 35.D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12(12):2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- 36.Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81(6):392–394. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- 37.Ritchie HH, Berry JE, Somerman MJ, Hanks CT, Bronckers AL, Hotton D, Papagerakis P, Berdal A, Butler WT. Dentin sialoprotein (DSP) transcripts: developmentally-sustained expression in odontoblasts and transient expression in pre-ameloblasts. Eur J Oral Sci. 1997;105(5 Pt 1):405–413. doi: 10.1111/j.1600-0722.1997.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 38.Bleicher R, Couble ML, Farges JC, Couble P, Magloire H. Sequential expression of matrix protein genes in developing rat teeth. Matrix Biol. 1999;18(2):133–143. doi: 10.1016/s0945-053x(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 39.Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc Natl Acad Sci U S A. 2001;98(8):4516–4521. doi: 10.1073/pnas.081075198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magne D, Pilet P, Weiss P, Daculsi G. Fourier transform infrared microspectroscopic investigation of the maturation of nonstoichiometric apatites in mineralized tissues: a horse dentin study. Bone. 2001;29(6):547–552. doi: 10.1016/s8756-3282(01)00609-3. [DOI] [PubMed] [Google Scholar]
- 41.Unterbrink A, O’Sullivan M, Chen S, MacDougall M. TGF beta-1 downregulates DMP-1 and DSPP in odontoblasts. Connect Tissue Res. 2002;43:2–3. 354–358. doi: 10.1080/03008200290000565. [DOI] [PubMed] [Google Scholar]
- 42.Bei M, Kratochwil K, Maas RL. BMP4 rescues a non-cell-autonomous function of Msxl in tooth development. Development. 2000;127(21):4711–4718. doi: 10.1242/dev.127.21.4711. [DOI] [PubMed] [Google Scholar]
- 43.Nakashima M, Nagasawa H, Yamada Y, Reddi AH. Regulatory role of transforming growth factor-beta, bone morphogenetic protein-2, and protein-4 on gene expression of extracellular matrix proteins and differentiation of dental pulp cells. Dev Biol. 1994;162(1):18–28. doi: 10.1006/dbio.1994.1063. [DOI] [PubMed] [Google Scholar]
- 44.Reddi AH, Cunningham NS. Initiation and promotion of bone differentiation by bone morphogenetic proteins. J Bone Miner Res. 1993;8(Suppl 2):8499–502. doi: 10.1002/jbmr.5650081313. [DOI] [PubMed] [Google Scholar]


