Abstract
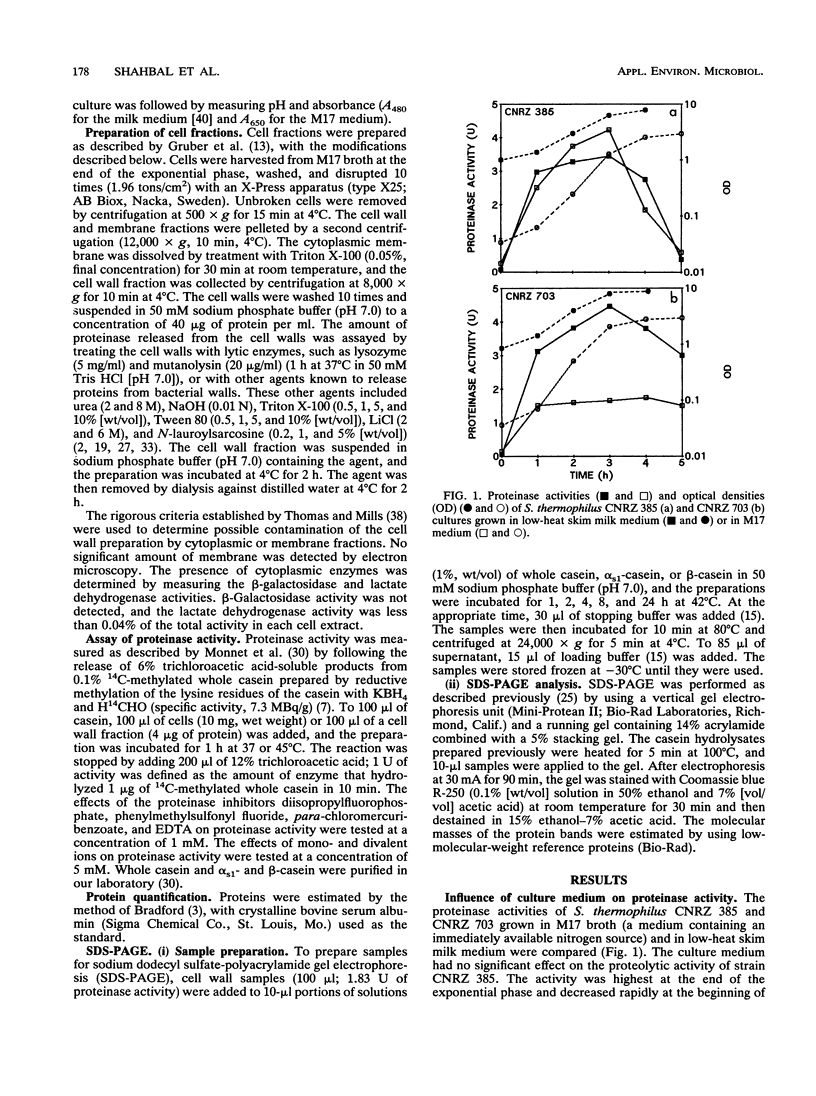
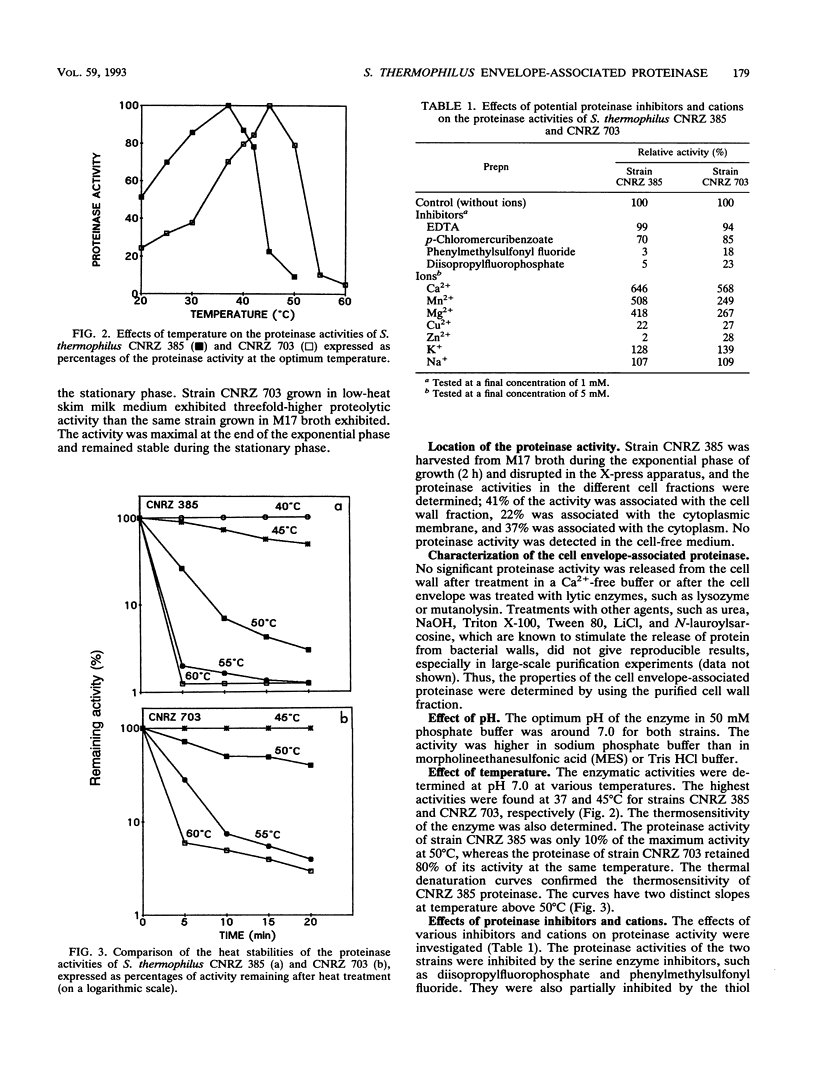
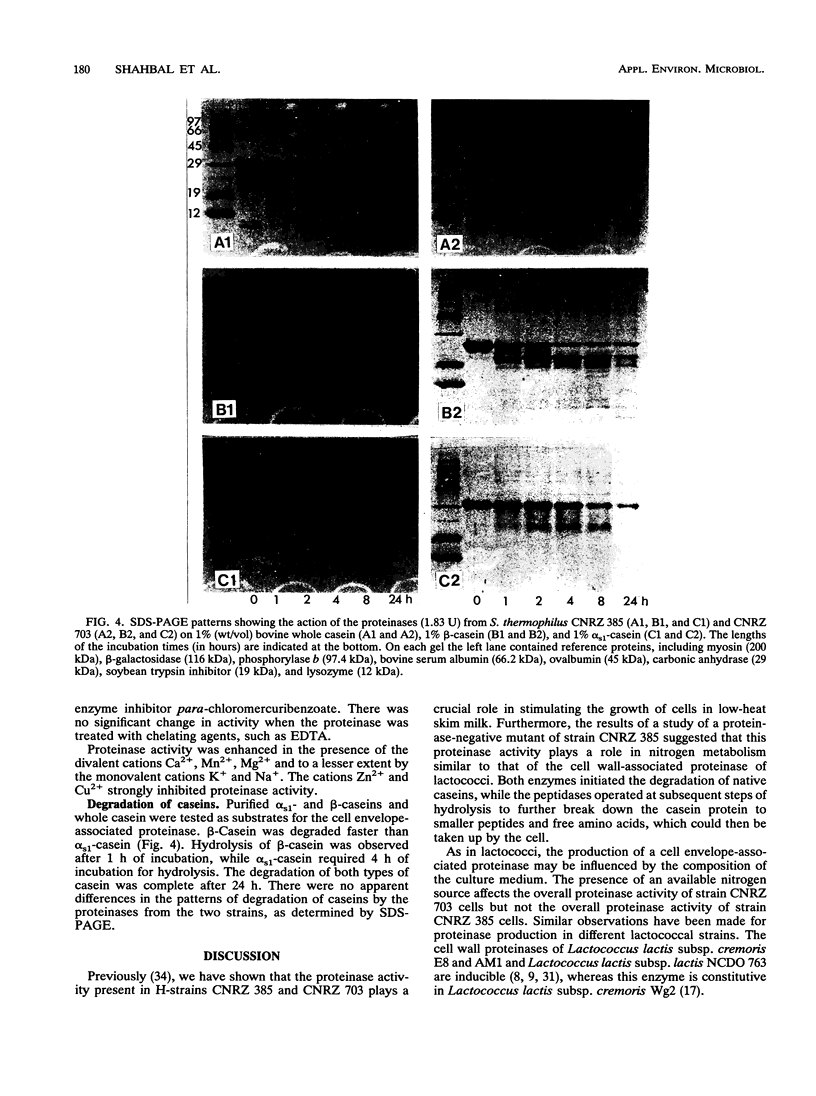
The production and biochemical properties of cell envelope-associated proteinases from two strains of Streptococcus thermophilus (strains CNRZ 385 and CNRZ 703) were compared. No significant difference in proteinase activity was found for strain CNRZ 385 when cells were grown in skim milk medium and M17 broth. Strain CNRZ 703 exhibited a threefold-higher proteinase activity when cells were grown in low-heat skim milk medium than when grown in M17 broth. Forty-one percent of the total activity of CNRZ 385 was localized on the cell wall. The optimum pH for enzymatic activity at 37°C was around 7.0. Serine proteinase inhibitors, such as phenylmethylsulfonyl fluoride and diisopropylfluorophosphate, inhibited the enzyme activity in both strains. The divalents cations Ca2+, Mg2+, and Mn2+ were activators, while Zn2+ and Cu2+ were inhibitors. β-Casein was hydrolyzed more rapidly than αs1-casein. The results of DNA hybridization and immunoblot studies suggested that the S. thermophilus cell wall proteinase and the lactococcal proteinase are not closely related.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyle P. J., Mathison G. E., Chandan R. C. Production of cell-bound proteinase by Lactobacillus bulgaricus and its location in the bacterial cell. J Appl Bacteriol. 1976 Aug;41(1):175–184. doi: 10.1111/j.1365-2672.1976.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Bergère J. L., Hayes H., Commissaire J. Major protein components in the cell envelope of Clostridium tyrobutyricum. Ann Inst Pasteur Microbiol. 1986 Nov-Dec;137B(3):271–282. doi: 10.1016/s0769-2609(86)80117-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Cleary P. P. Cloning and expression of the streptococcal C5a peptidase gene in Escherichia coli: linkage to the type 12 M protein gene. Infect Immun. 1989 Jun;57(6):1740–1745. doi: 10.1128/iai.57.6.1740-1745.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmin C., Pebay M., Simonet J. M., Decaris B. A species-specific DNA probe obtained from Streptococcus salivarius subsp. thermophilus detects strain restriction polymorphism. FEMS Microbiol Lett. 1991 Jun 15;65(2):123–128. doi: 10.1016/0378-1097(91)90290-q. [DOI] [PubMed] [Google Scholar]
- Donnelly W. J., Barry J. G., Richardson T. 14C-Methylated beta-casein as a substrate for plasmin, and its application to the study of milk protein transformations. Biochim Biophys Acta. 1980 Nov 20;626(1):117–126. doi: 10.1016/0005-2795(80)90203-2. [DOI] [PubMed] [Google Scholar]
- Exterkate F. A., de Veer G. J. Partial Isolation and Degradation of Caseins by Cell Wall Proteinase(s) of Streptococcus cremoris HP. Appl Environ Microbiol. 1985 Feb;49(2):328–332. doi: 10.1128/aem.49.2.328-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haandrikman A. J., Meesters R., Laan H., Konings W. N., Kok J., Venema G. Processing of the lactococcal extracellular serine proteinase. Appl Environ Microbiol. 1991 Jul;57(7):1899–1904. doi: 10.1128/aem.57.7.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., van Sinderen D., Kok J., Konings W. N. Cell Wall-Associated Proteases of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1987 Apr;53(4):853–859. doi: 10.1128/aem.53.4.853-859.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyama R., Massidda O., Daneo-Moore L., Shockman G. D. Properties of cell wall-associated DD-carboxypeptidase of Enterococcus hirae (Streptococcus faecium) ATCC 9790 extracted with alkali. J Bacteriol. 1990 Jul;172(7):3718–3724. doi: 10.1128/jb.172.7.3718-3724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic M., Fira D., Banina A., Topisirovic L. Characterization of the Cell Wall-Bound Proteinase of Lactobacillus casei HN14. Appl Environ Microbiol. 1991 Jun;57(6):1753–1757. doi: 10.1128/aem.57.6.1753-1757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Kok J., Leenhouts K. J., Haandrikman A. J., Ledeboer A. M., Venema G. Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1988 Jan;54(1):231–238. doi: 10.1128/aem.54.1.231-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok J., Venema G. Genetics of proteinases of lactic acid bacteria. Biochimie. 1988 Apr;70(4):475–488. doi: 10.1016/0300-9084(88)90084-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laloi P., Atlan D., Blanc B., Gilbert C., Portalier R. Cell-wall-associated proteinase of Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397: differential extraction, purification and properties of the enzyme. Appl Microbiol Biotechnol. 1991 Nov;36(2):196–204. doi: 10.1007/BF00164419. [DOI] [PubMed] [Google Scholar]
- Monnet V., Le Bars D., Gripon J. C. Purification and characterization of a cell wall proteinase from Streptococcus lactis NCDO 763. J Dairy Res. 1987 May;54(2):247–255. doi: 10.1017/s0022029900025383. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser S., Exterkate F. A., Slangen C. J., de Veer G. J. Comparative Study of Action of Cell Wall Proteinases from Various Strains of Streptococcus cremoris on Bovine alpha(s1)-, beta-, and kappa-Casein. Appl Environ Microbiol. 1986 Nov;52(5):1162–1166. doi: 10.1128/aem.52.5.1162-1166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]