Abstract
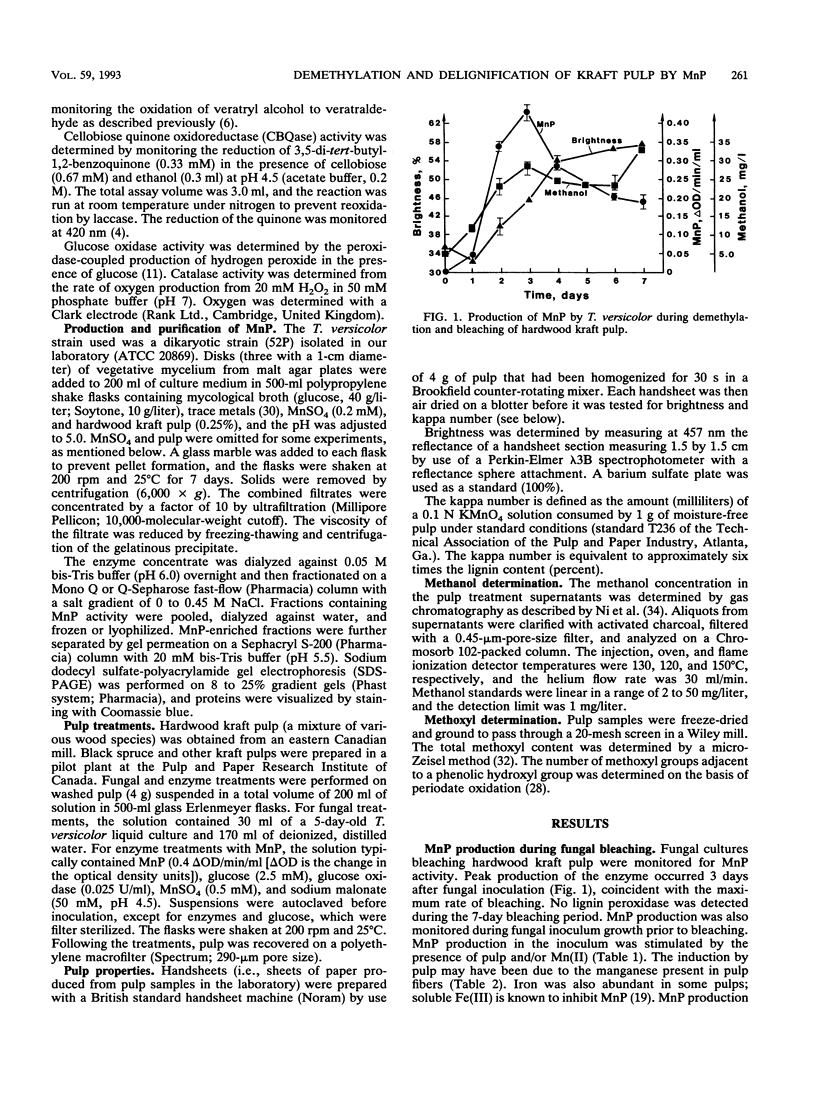
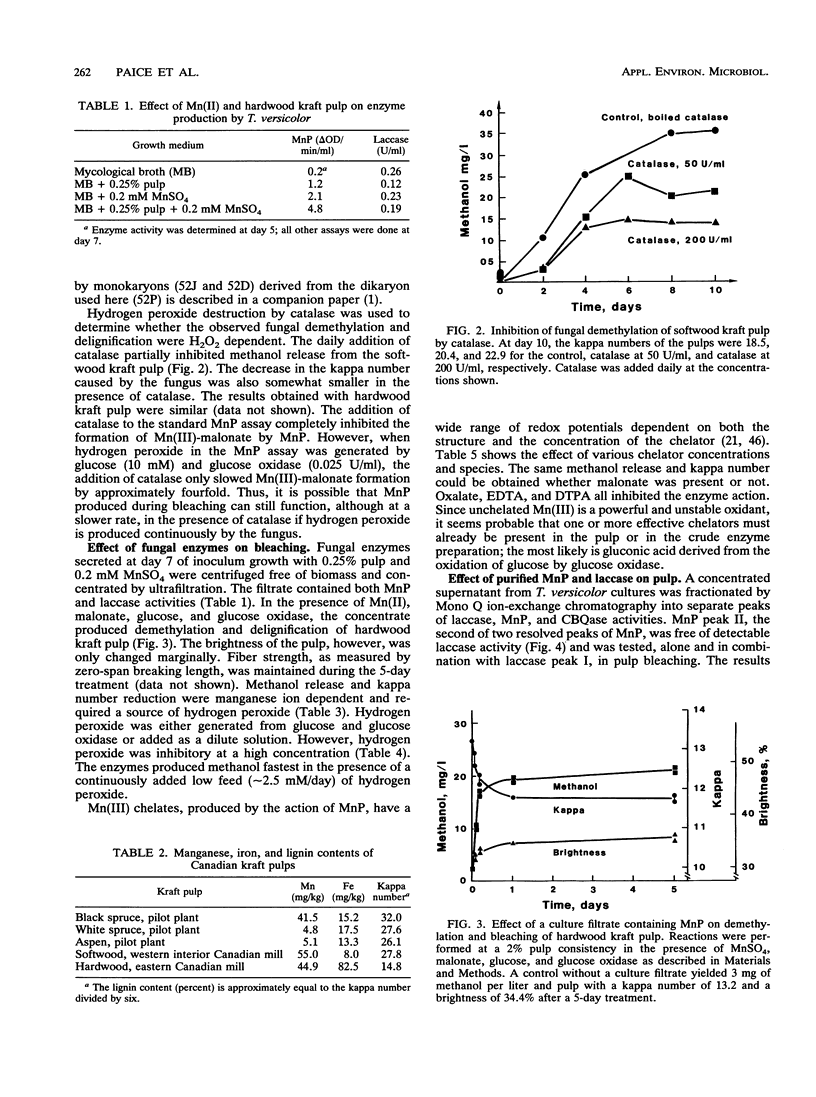
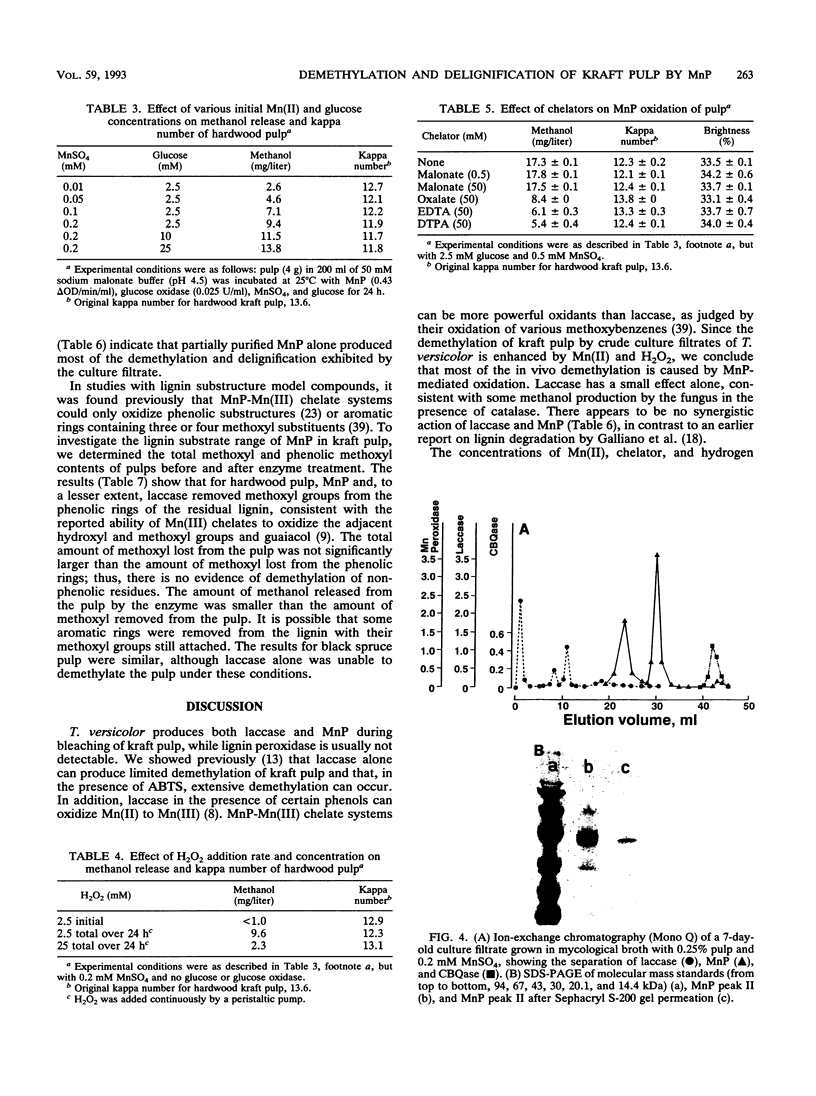
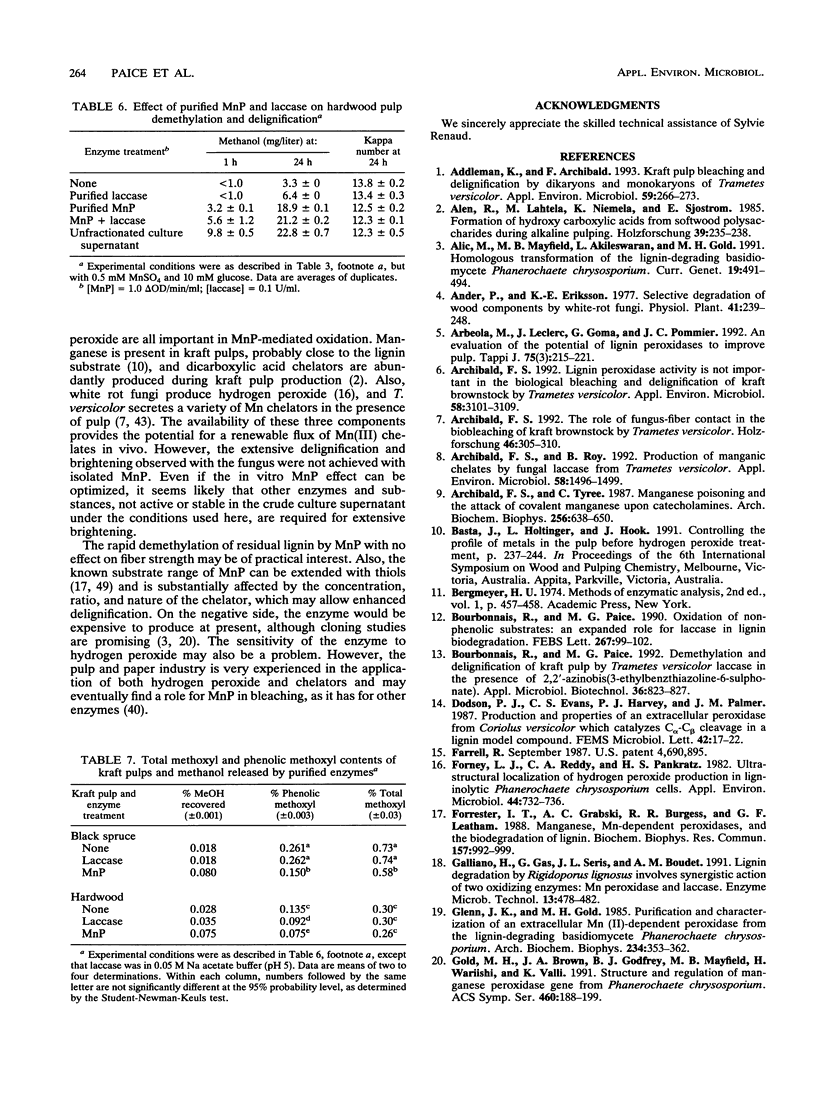
Previous work has shown that Trametes (Coriolus) versicolor bleaches kraft pulp brownstock with the concomitant release of methanol. In this work, the fungus is shown to produce both laccase and manganese peroxidase (MnP) but not lignin peroxidase during pulp bleaching. MnP production was enhanced by the presence of pulp and/or Mn(II) ions. The maximum level of secreted MnP was coincident with the maximum rate of fungal bleaching. Culture filtrates isolated from bleaching cultures produced Mn(II)- and hydrogen peroxide-dependent pulp demethylation and delignification. Laccase and MnP were separated by ion-exchange chromatography. Purified MnP alone produced most of the demethylation and delignification exhibited by the culture filtrates. On the basis of the methanol released and the total and phenolic methoxyl contents of the pulp, it appears that MnP shows a preference for the oxidation of phenolic lignin substructures. The extensive increase in brightness observed in the fungus-treated pulp was not found with MnP alone. Therefore, either the MnP effect must be optimized or other enzymes or compounds from the fungus are also required for brightening.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addleman K., Archibald F. Kraft Pulp Bleaching and Delignification by Dikaryons and Monokaryons of Trametes versicolor. Appl Environ Microbiol. 1993 Jan;59(1):266–273. doi: 10.1128/aem.59.1.266-273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S. Lignin Peroxidase Activity Is Not Important in Biological Bleaching and Delignification of Unbleached Kraft Pulp by Trametes versicolor. Appl Environ Microbiol. 1992 Sep;58(9):3101–3109. doi: 10.1128/aem.58.9.3101-3109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Tyree C. Manganese poisoning and the attack of trivalent manganese upon catecholamines. Arch Biochem Biophys. 1987 Aug 1;256(2):638–650. doi: 10.1016/0003-9861(87)90621-7. [DOI] [PubMed] [Google Scholar]
- Archibald F., Roy B. Production of manganic chelates by laccase from the lignin-degrading fungus Trametes (Coriolus) versicolor. Appl Environ Microbiol. 1992 May;58(5):1496–1499. doi: 10.1128/aem.58.5.1496-1499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M. G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990 Jul 2;267(1):99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Pankratz H. S. Ultrastructural Localization of Hydrogen Peroxide Production in Ligninolytic Phanerochaete chrysosporium Cells. Appl Environ Microbiol. 1982 Sep;44(3):732–736. doi: 10.1128/aem.44.3.732-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester I. T., Grabski A. C., Burgess R. R., Leatham G. F. Manganese, Mn-dependent peroxidases, and the biodegradation of lignin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):992–999. doi: 10.1016/s0006-291x(88)80972-0. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Lackner R., Srebotnik E., Messner K. Oxidative degradation of high molecular weight chlorolignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1092–1098. doi: 10.1016/0006-291x(91)91004-v. [DOI] [PubMed] [Google Scholar]
- Mandels M., Andreotti R., Roche C. Measurement of saccharifying cellulase. Biotechnol Bioeng Symp. 1976;(6):21–33. [PubMed] [Google Scholar]
- Michel F. C., Jr, Dass S. B., Grulke E. A., Reddy C. A. Role of manganese peroxidases and lignin peroxidases of Phanerochaete chrysosporium in the decolorization of kraft bleach plant effluent. Appl Environ Microbiol. 1991 Aug;57(8):2368–2375. doi: 10.1128/aem.57.8.2368-2375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp J. L., Kirk T. K. Oxidation of methoxybenzenes by manganese peroxidase and by Mn3+. Arch Biochem Biophys. 1991 Jul;288(1):145–148. doi: 10.1016/0003-9861(91)90176-j. [DOI] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tuor U., Wariishi H., Schoemaker H. E., Gold M. H. Oxidation of phenolic arylglycerol beta-aryl ether lignin model compounds by manganese peroxidase from Phanerochaete chrysosporium: oxidative cleavage of an alpha-carbonyl model compound. Biochemistry. 1992 Jun 2;31(21):4986–4995. doi: 10.1021/bi00136a011. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Renganathan V., Gold M. H. Thiol-mediated oxidation of nonphenolic lignin model compounds by manganese peroxidase of Phanerochaete chrysosporium. J Biol Chem. 1989 Aug 25;264(24):14185–14191. [PubMed] [Google Scholar]



