Abstract
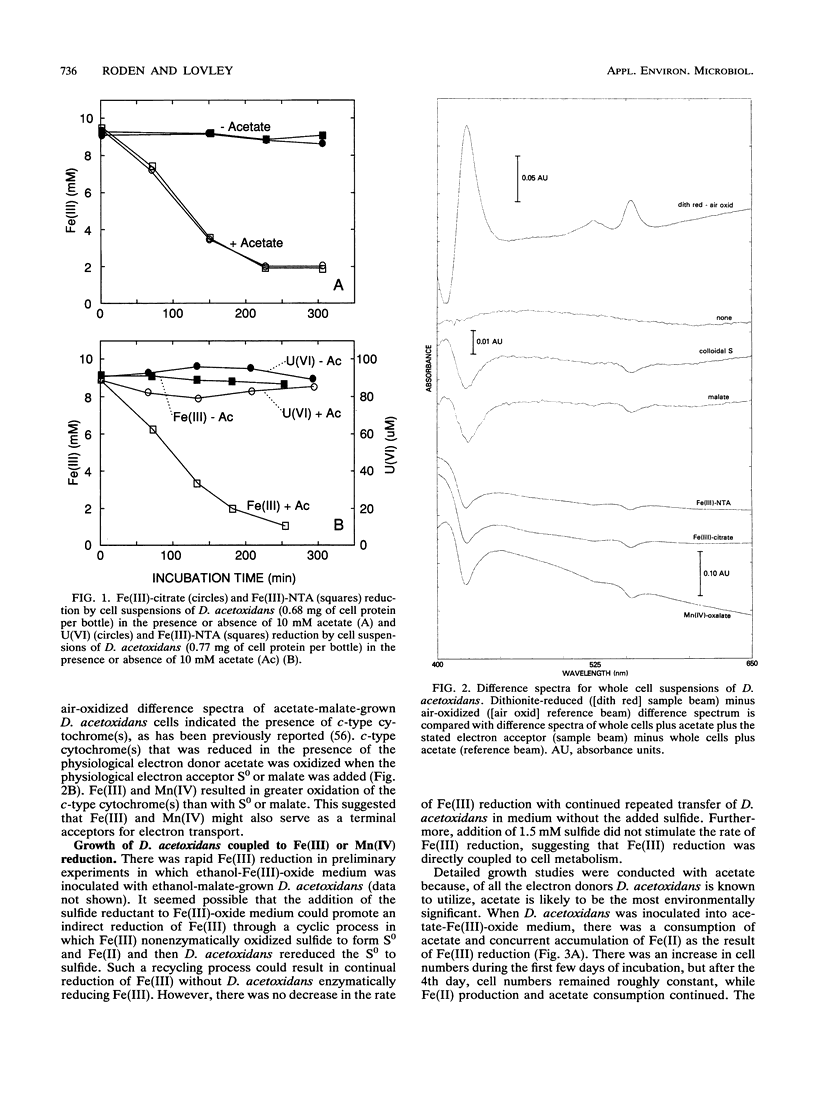
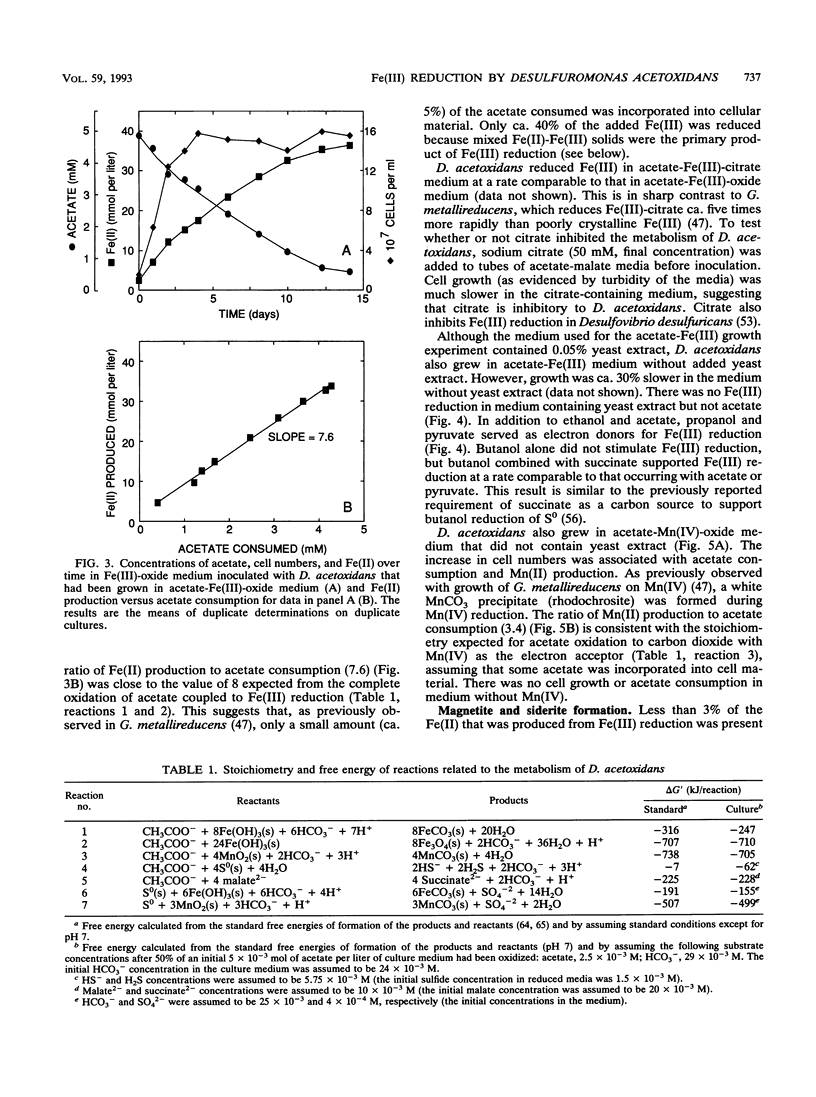
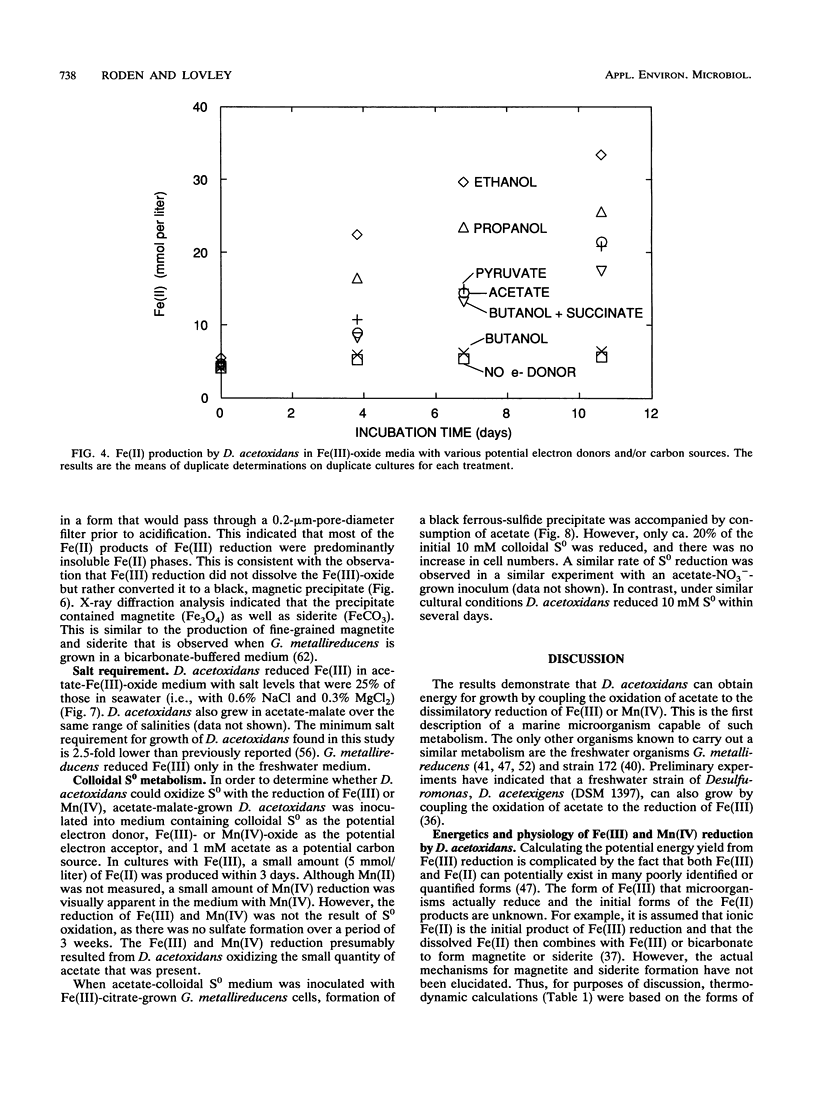
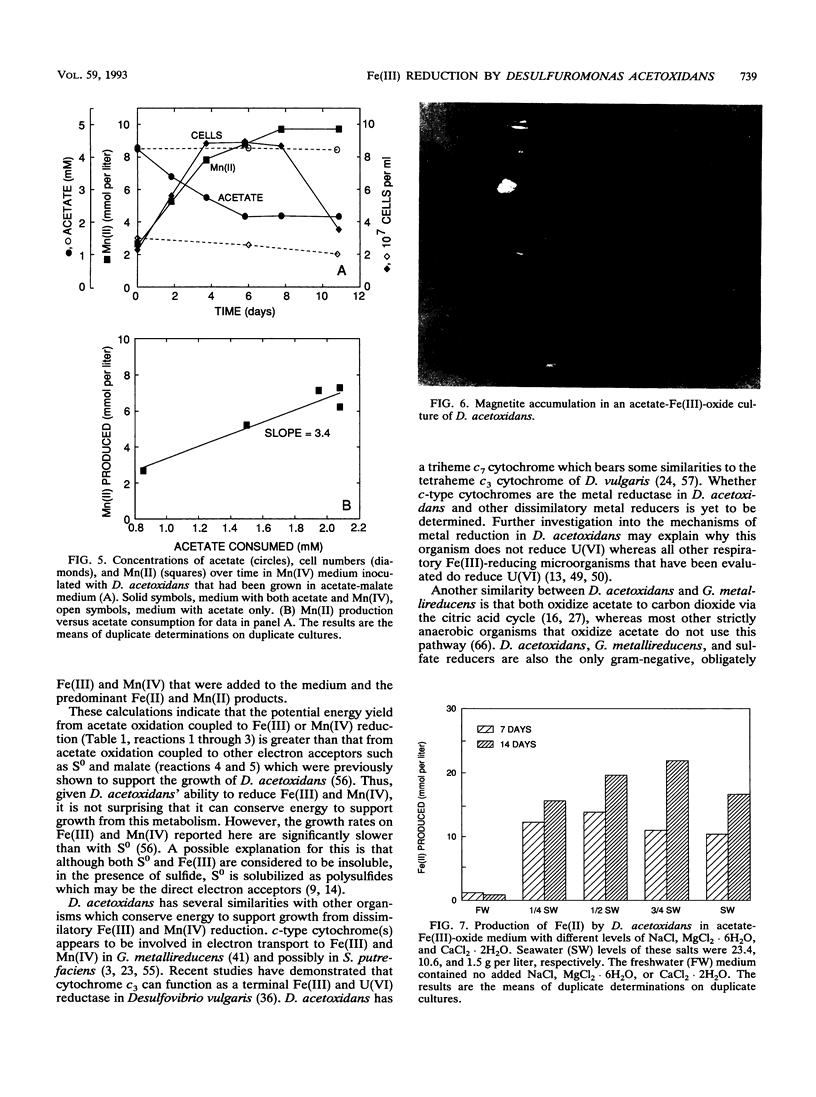
The ability of the marine microorganism Desulfuromonas acetoxidans to reduce Fe(III) was investigated because of its close phylogenetic relationship with the freshwater dissimilatory Fe(III) reducer Geobacter metallireducens. Washed cell suspensions of the type strain of D. acetoxidans reduced soluble Fe(III)-citrate and Fe(III) complexed with nitriloacetic acid. The c-type cytochrome(s) of D. acetoxidans was oxidized by Fe(III)-citrate and Mn(IV)-oxalate, as well as by two electron acceptors known to support growth, colloidal sulfur and malate. D. acetoxidans grew in defined anoxic, bicarbonate-buffered medium with acetate as the sole electron donor and poorly crystalline Fe(III) or Mn(IV) as the sole electron acceptor. Magnetite (Fe3O4) and siderite (FeCO3) were the major end products of Fe(III) reduction, whereas rhodochrosite (MnCO3) was the end product of Mn(IV) reduction. Ethanol, propanol, pyruvate, and butanol also served as electron donors for Fe(III) reduction. In contrast to D. acetoxidans, G. metallireducens could only grow in freshwater medium and it did not conserve energy to support growth from colloidal S0 reduction. D. acetoxidans is the first marine microorganism shown to conserve energy to support growth by coupling the complete oxidation of organic compounds to the reduction of Fe(III) or Mn(IV). Thus, D. acetoxidans provides a model enzymatic mechanism for Fe(III) or Mn(IV) oxidation of organic compounds in marine and estuarine sediments. These findings demonstrate that 16S rRNA phylogenetic analyses can suggest previously unrecognized metabolic capabilities of microorganisms.
Full text
PDF

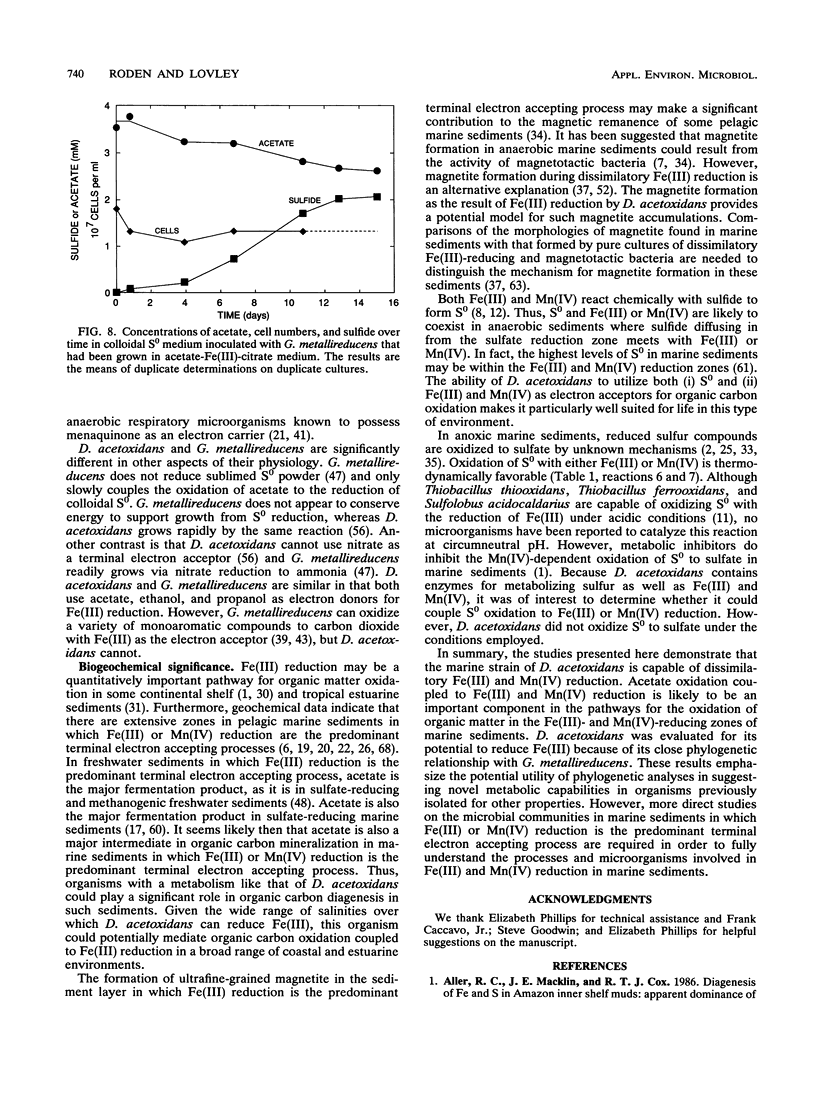


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. G., DiChristina T. J., Hoffmann M. R. Inhibitor studies of dissimilative Fe(III) reduction by Pseudomonas sp. strain 200 ("Pseudomonas ferrireductans") Appl Environ Microbiol. 1986 Aug;52(2):281–289. doi: 10.1128/aem.52.2.281-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumentals I. I., Itoh M., Olson G. J., Kelly R. M. Role of Polysulfides in Reduction of Elemental Sulfur by the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 May;56(5):1255–1262. doi: 10.1128/aem.56.5.1255-1262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Gustafson J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl Environ Microbiol. 1976 Oct;32(4):567–571. doi: 10.1128/aem.32.4.567-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccavo F., Blakemore R. P., Lovley D. R. A Hydrogen-Oxidizing, Fe(III)-Reducing Microorganism from the Great Bay Estuary, New Hampshire. Appl Environ Microbiol. 1992 Oct;58(10):3211–3216. doi: 10.1128/aem.58.10.3211-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield D. E. Reactive iron in marine sediments. Geochim Cosmochim Acta. 1989;53:619–632. doi: 10.1016/0016-7037(89)90005-7. [DOI] [PubMed] [Google Scholar]
- Champine J. E., Goodwin S. Acetate catabolism in the dissimilatory iron-reducing isolate GS-15. J Bacteriol. 1991 Apr;173(8):2704–2706. doi: 10.1128/jb.173.8.2704-2706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C. C., Phillips D. I., Lazarus J. H., Parkes A. B. Effect of low dose iodide supplementation on thyroid function in potentially susceptible subjects: are dietary iodide levels in Britain acceptable? Clin Endocrinol (Oxf) 1991 May;34(5):413–416. doi: 10.1111/j.1365-2265.1991.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981 Jun;45(2):316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiechtner M. D., Kassner R. J. The redox properties and heme environment of cytochrome c-551.5 from Desulfuromonas acetoxidans. Biochim Biophys Acta. 1979 Aug 28;579(2):269–278. doi: 10.1016/0005-2795(79)90054-0. [DOI] [PubMed] [Google Scholar]
- Jørgensen B. B. A thiosulfate shunt in the sulfur cycle of marine sediments. Science. 1990 Jul 13;249(4965):152–154. doi: 10.1126/science.249.4965.152. [DOI] [PubMed] [Google Scholar]
- Lovley D. R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991 Jun;55(2):259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Greening R. C., Ferry J. G. Rapidly growing rumen methanogenic organism that synthesizes coenzyme M and has a high affinity for formate. Appl Environ Microbiol. 1984 Jul;48(1):81–87. doi: 10.1128/aem.48.1.81-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Lonergan D. J. Anaerobic Oxidation of Toluene, Phenol, and p-Cresol by the Dissimilatory Iron-Reducing Organism, GS-15. Appl Environ Microbiol. 1990 Jun;56(6):1858–1864. doi: 10.1128/aem.56.6.1858-1864.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal potomac river. Appl Environ Microbiol. 1986 Oct;52(4):751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J., Lonergan D. J. Hydrogen and Formate Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese by Alteromonas putrefaciens. Appl Environ Microbiol. 1989 Mar;55(3):700–706. doi: 10.1128/aem.55.3.700-706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988 Jun;54(6):1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol. 1987 Jul;53(7):1536–1540. doi: 10.1128/aem.53.7.1536-1540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol. 1992 Mar;58(3):850–856. doi: 10.1128/aem.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Requirement for a Microbial Consortium To Completely Oxidize Glucose in Fe(III)-Reducing Sediments. Appl Environ Microbiol. 1989 Dec;55(12):3234–3236. doi: 10.1128/aem.55.12.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W. Effects of medium composition on cell pigmentation, cytochrome content, and ferric iron reduction in a Pseudomonas sp. isolated from crude oil. Can J Microbiol. 1982 Aug;28(8):989–992. doi: 10.1139/m82-148. [DOI] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Probst I., Bruschi M., Pfennig N., Le Gall J. Cytochrome c-551.5 (c7) from Desulfuromonas acetoxidans. Biochim Biophys Acta. 1977 Apr 11;460(1):58–64. doi: 10.1016/0005-2728(77)90151-7. [DOI] [PubMed] [Google Scholar]
- Sørensen J., Christensen D., Jørgensen B. B. Volatile Fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981 Jul;42(1):5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982 Feb;43(2):319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Möller-Zinkhan D., Spormann A. M. Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Annu Rev Microbiol. 1989;43:43–67. doi: 10.1146/annurev.mi.43.100189.000355. [DOI] [PubMed] [Google Scholar]
- Tugel J. B., Hines M. E., Jones G. E. Microbial iron reduction by enrichment cultures isolated from estuarine sediments. Appl Environ Microbiol. 1986 Nov;52(5):1167–1172. doi: 10.1128/aem.52.5.1167-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



