Abstract
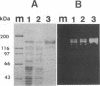
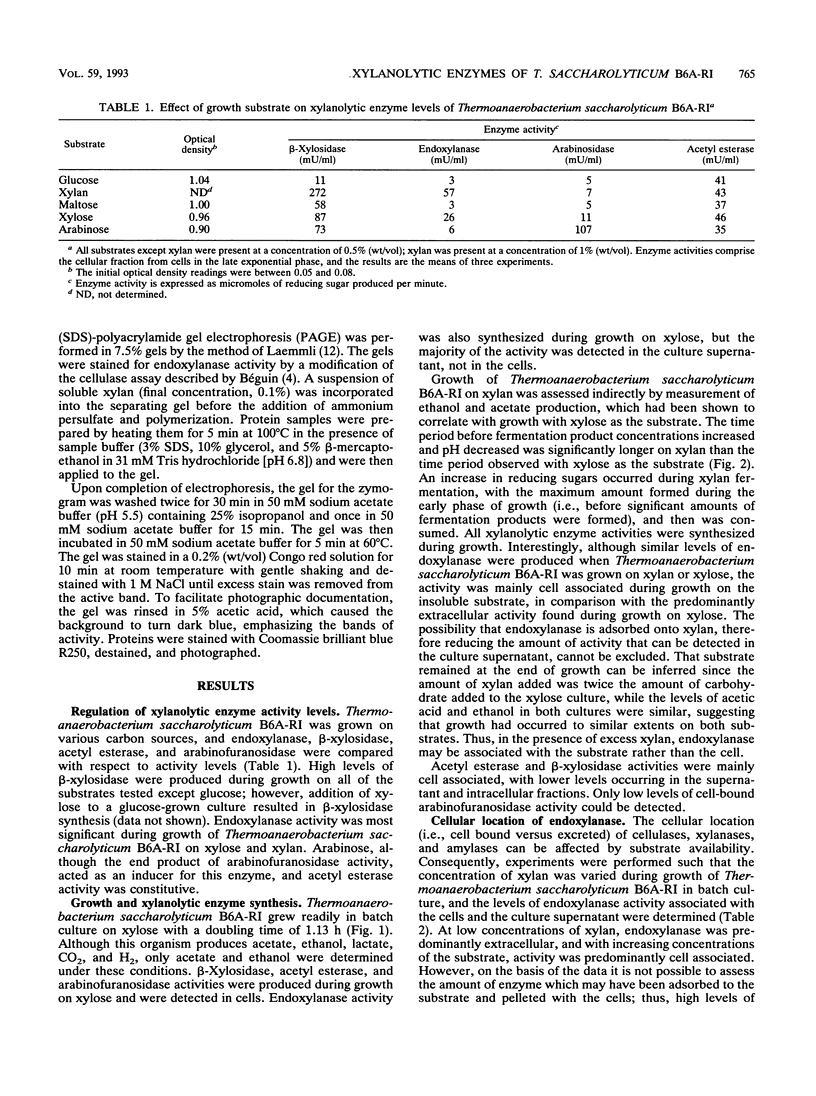
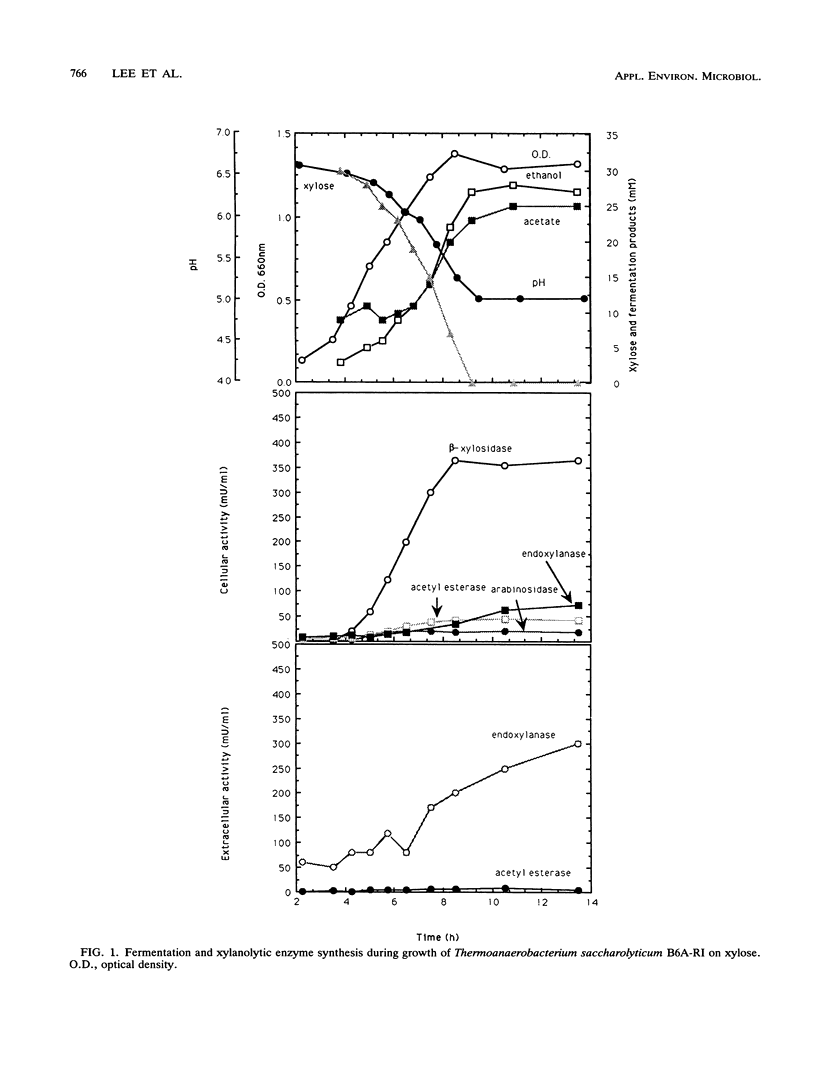
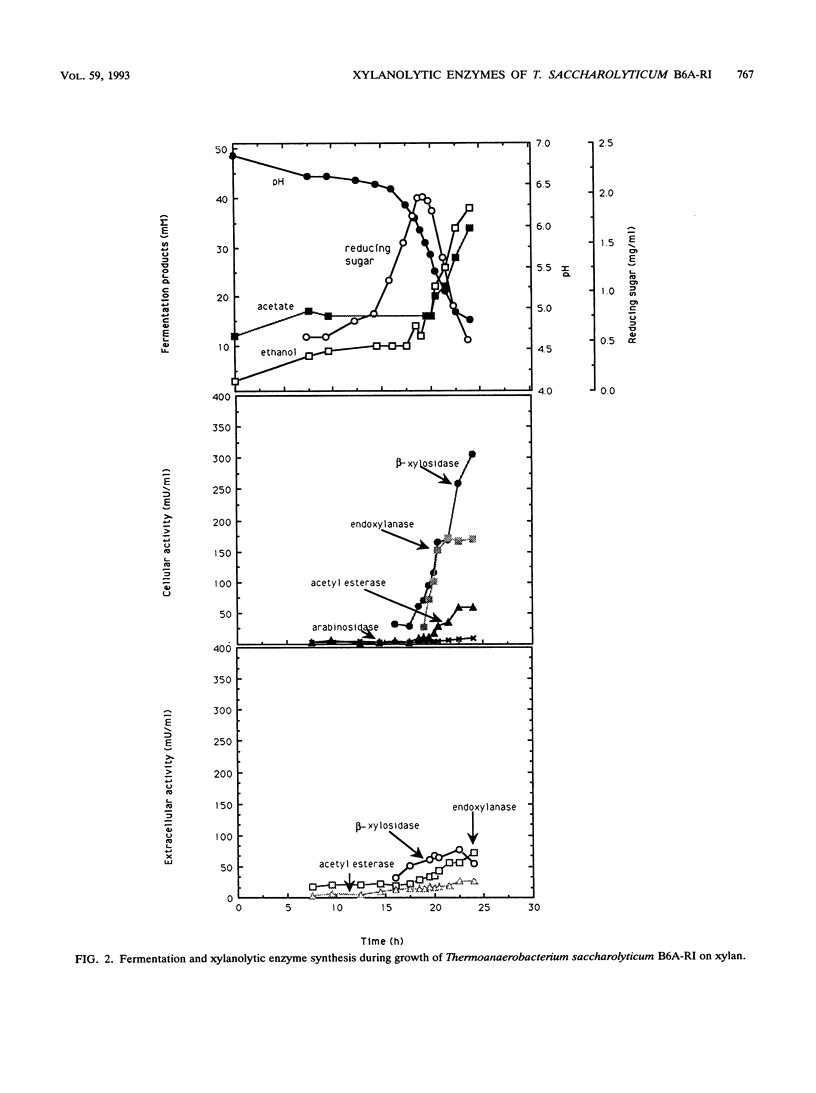
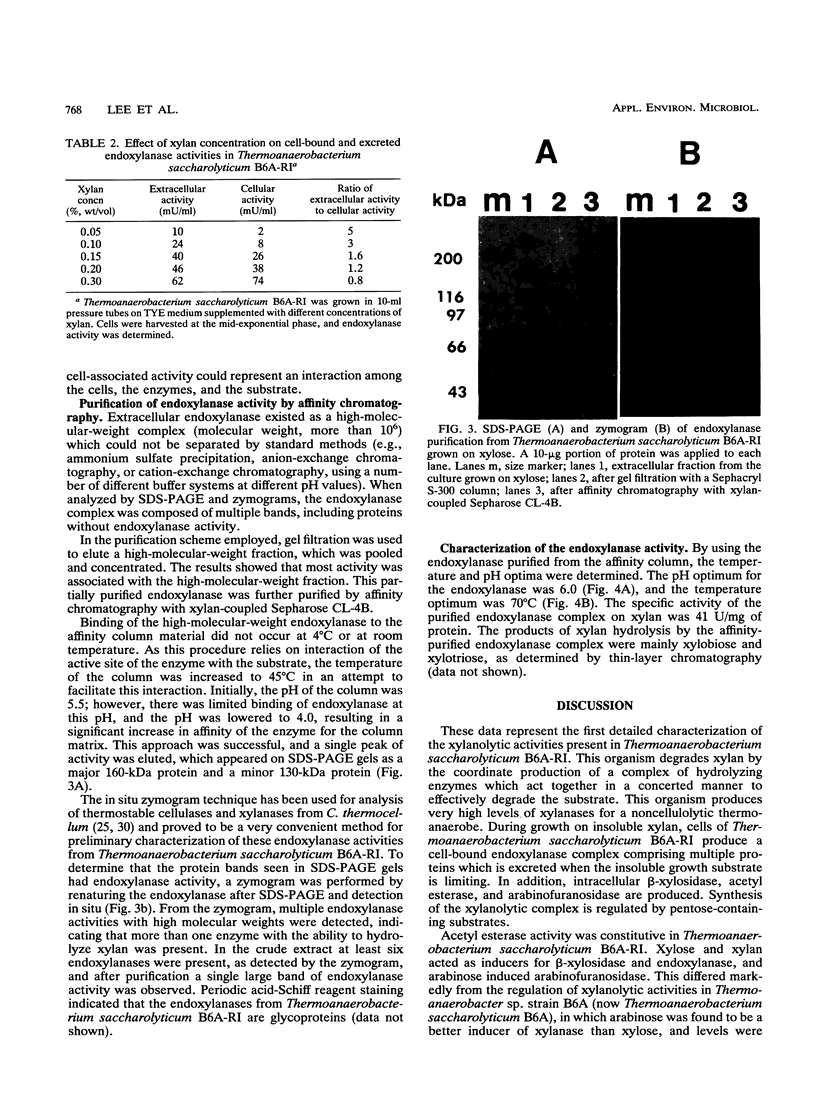
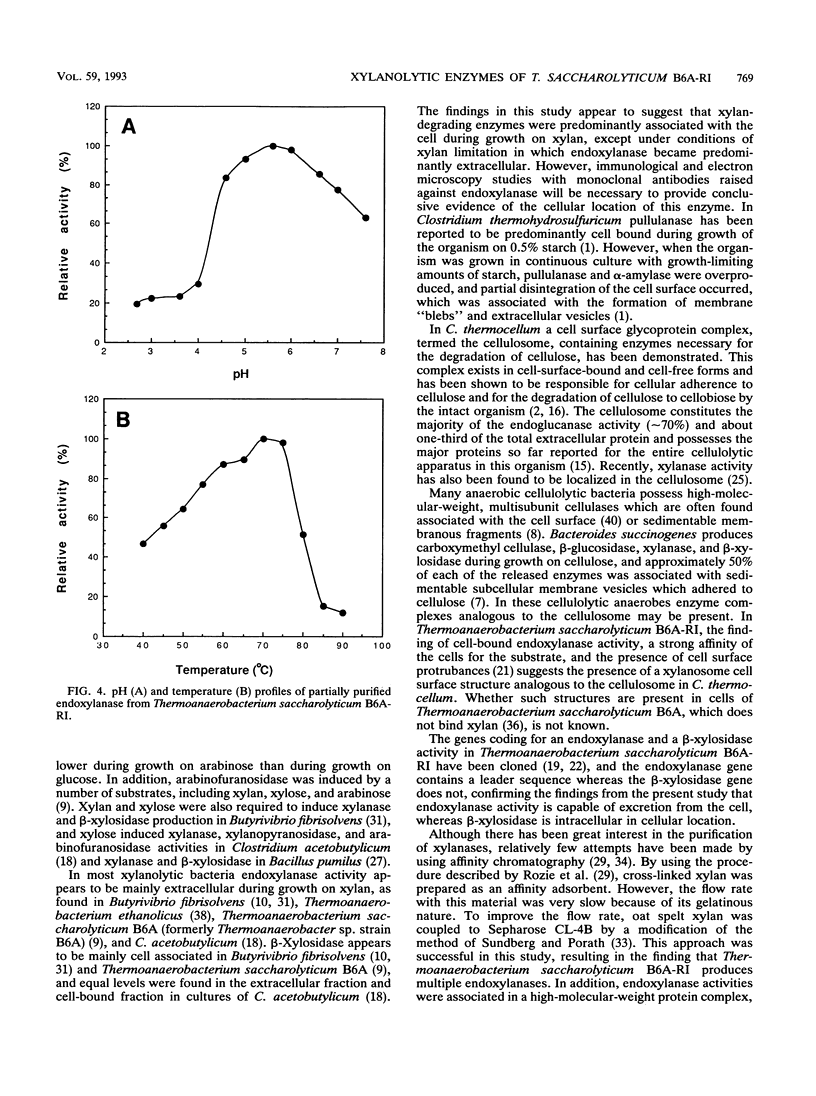
During growth on xylan and xylose Thermoanaerobacterium saccharolyticum B6A-RI produced endoxylanase, β-xylosidase, arabinofuranosidase, and acetyl esterase, and the first three activities appeared to be produced coordinately. During nonlimiting growth on xylan, these enzyme activities were predominantly cell associated; however, during growth on limiting concentrations of xylan, the majority of endoxylanase activity was extracellular rather than cell associated. Endoxylanase, β-xylosidase, and arabinofuranosidase activities were induced by xylan, xylose, and arabinose, respectively. Acetyl esterase activity was constitutive, and endoxylanase activity was catabolite repressed by glucose. Extracellular endoxylanase existed as a high-molecular-weight complex (molecular weight, more than 106). When analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and zymograms, the crude endoxylanase complex was composed of at least six activity bands. Endoxylanase was purified by gel filtration with Sephacryl S-300 and affinity chromatography with xylan coupled to Sepharose CL-4B preequilibrated to 45°C with 50 mM sodium acetate buffer (pH 4.0) and eluted with 0.1% soluble xylan. A single area of endoxylanase activity was identified on the zymogram; when this activity was analyzed by SDS-PAGE, it was composed of a major protein with a molecular weight of approximately 160,000 and a minor protein with a molecular weight of approximately 130,000. The endoxylanase activity stained with Schiff's reagent, indicative of glycoproteins, displayed a specific activity of 41 U/mg of protein on xylan, and had pH and temperature optima of 6.0 and 70°C, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Lamed R. Ultrastructure of the cell surface cellulosome of Clostridium thermocellum and its interaction with cellulose. J Bacteriol. 1986 Sep;167(3):828–836. doi: 10.1128/jb.167.3.828-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Setter E., Lamed R. Organization and distribution of the cellulosome in Clostridium thermocellum. J Bacteriol. 1985 Aug;163(2):552–559. doi: 10.1128/jb.163.2.552-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Wolf R., Bothast R. J. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl Environ Microbiol. 1987 Dec;53(12):2849–2853. doi: 10.1128/aem.53.12.2849-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C., Saha B. C., Zeikus J. G. Characterization of thermoanaerobacter glucose isomerase in relation to saccharidase synthesis and development of single-step processes for sweetener production. Appl Environ Microbiol. 1990 Sep;56(9):2895–2901. doi: 10.1128/aem.56.9.2895-2901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Gibbins L. N. Xylanolytic Activity of Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Oct;50(4):1068–1076. doi: 10.1128/aem.50.4.1068-1076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Christian R., Kolbe J., Schulz G., Sleytr U. B. Analysis of a novel linkage unit of O-linked carbohydrates from the crystalline surface layer glycoprotein of Clostridium thermohydrosulfuricum S102-70. J Bacteriol. 1992 Apr;174(7):2236–2240. doi: 10.1128/jb.174.7.2236-2240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morag E., Bayer E. A., Lamed R. Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J Bacteriol. 1990 Oct;172(10):6098–6105. doi: 10.1128/jb.172.10.6098-6105.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly P. J. Xylanases: structure and function. Basic Life Sci. 1981;18:111–129. doi: 10.1007/978-1-4684-3980-9_8. [DOI] [PubMed] [Google Scholar]
- Schwarz W. H., Bronnenmeier K., Gräbnitz F., Staudenbauer W. L. Activity staining of cellulases in polyacrylamide gels containing mixed linkage beta-glucans. Anal Biochem. 1987 Jul;164(1):72–77. doi: 10.1016/0003-2697(87)90369-1. [DOI] [PubMed] [Google Scholar]
- Sewell G. W., Aldrich H. C., Williams D., Mannarelli B., Wilkie A., Hespell R. B., Smith P. H., Ingram L. O. Isolation and Characterization of Xylan-Degrading Strains of Butyrivibrio fibrisolvens from a Napier Grass-Fed Anaerobic Digester. Appl Environ Microbiol. 1988 May;54(5):1085–1090. doi: 10.1128/aem.54.5.1085-1090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sundberg L., Porath J. Preparation of adsorbents for biospecific affinity chromatography. Attachment of group-containing ligands to insoluble polymers by means of bifuctional oxiranes. J Chromatogr. 1974 Mar 13;90(1):87–98. doi: 10.1016/s0021-9673(01)94777-6. [DOI] [PubMed] [Google Scholar]
- Ward O. P., Moo-Young M. Enzymatic degradation of cell wall and related plant polysaccharides. Crit Rev Biotechnol. 1989;8(4):237–274. doi: 10.3109/07388558909148194. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Wagner L. W., Knowlton S., Ng T. K. Thermophilic anaerobic bacteria which ferment hemicellulose: characterization of organisms and identification of plasmids. Arch Microbiol. 1984 May;138(1):31–36. doi: 10.1007/BF00425403. [DOI] [PubMed] [Google Scholar]
- Wiegel J., Mothershed C. P., Puls J. Differences in Xylan Degradation by Various Noncellulolytic Thermophilic Anaerobes and Clostridium thermocellum. Appl Environ Microbiol. 1985 Mar;49(3):656–659. doi: 10.1128/aem.49.3.656-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M., Wilson C. A., Stewart C. S. Preparation of the cellulase from the cellulolytic anaerobic rumen bacterium Ruminococcus albus and its release from the bacterial cell wall. Biochem J. 1982 Jul 1;205(1):129–137. doi: 10.1042/bj2050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]