Abstract
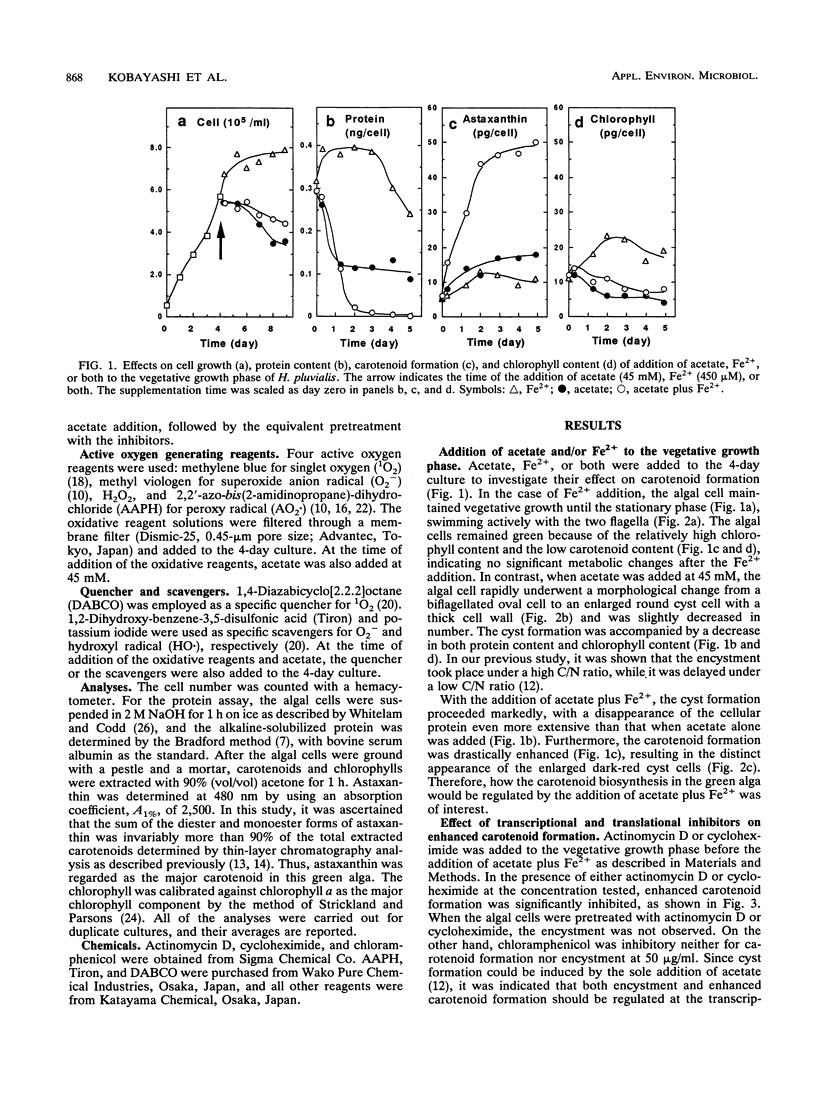
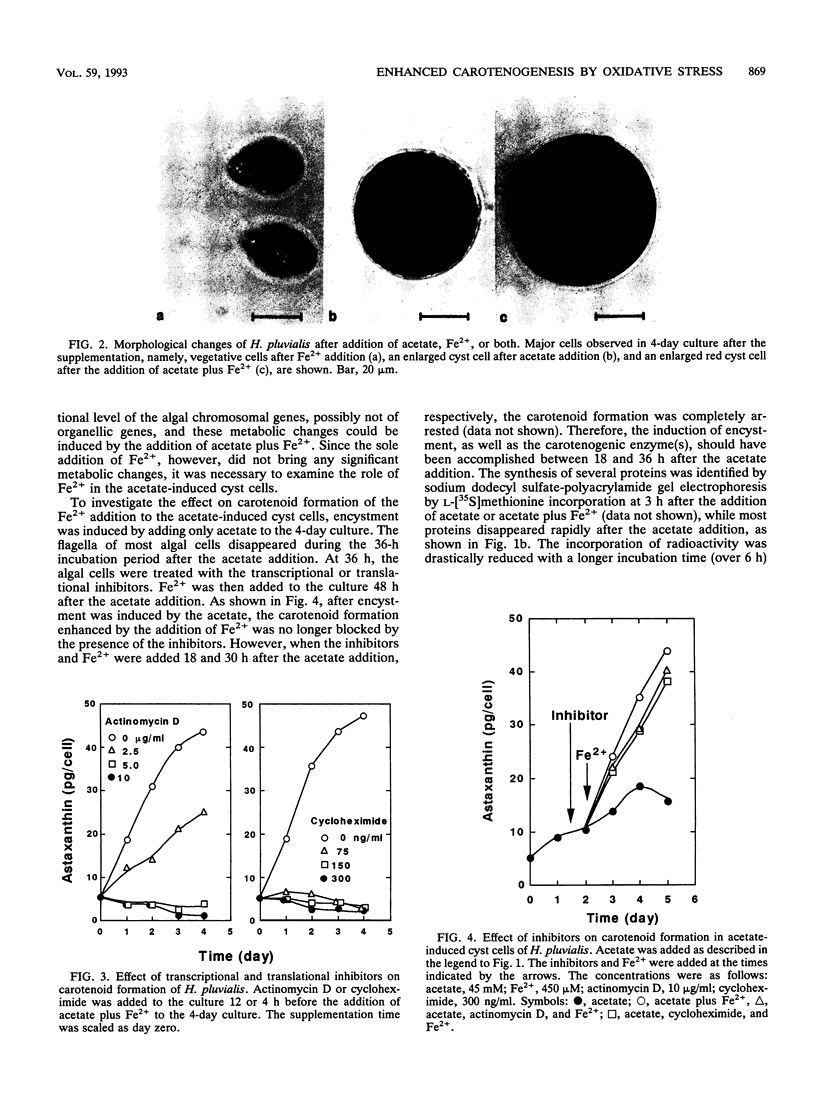
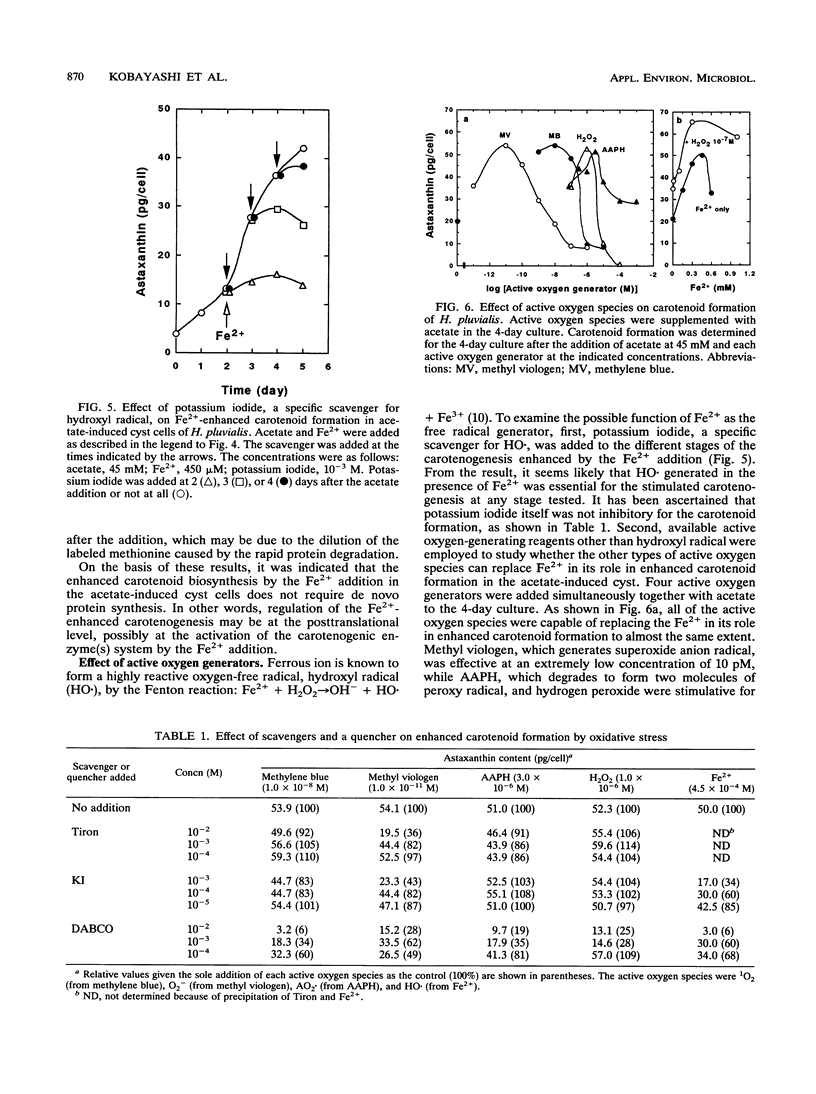
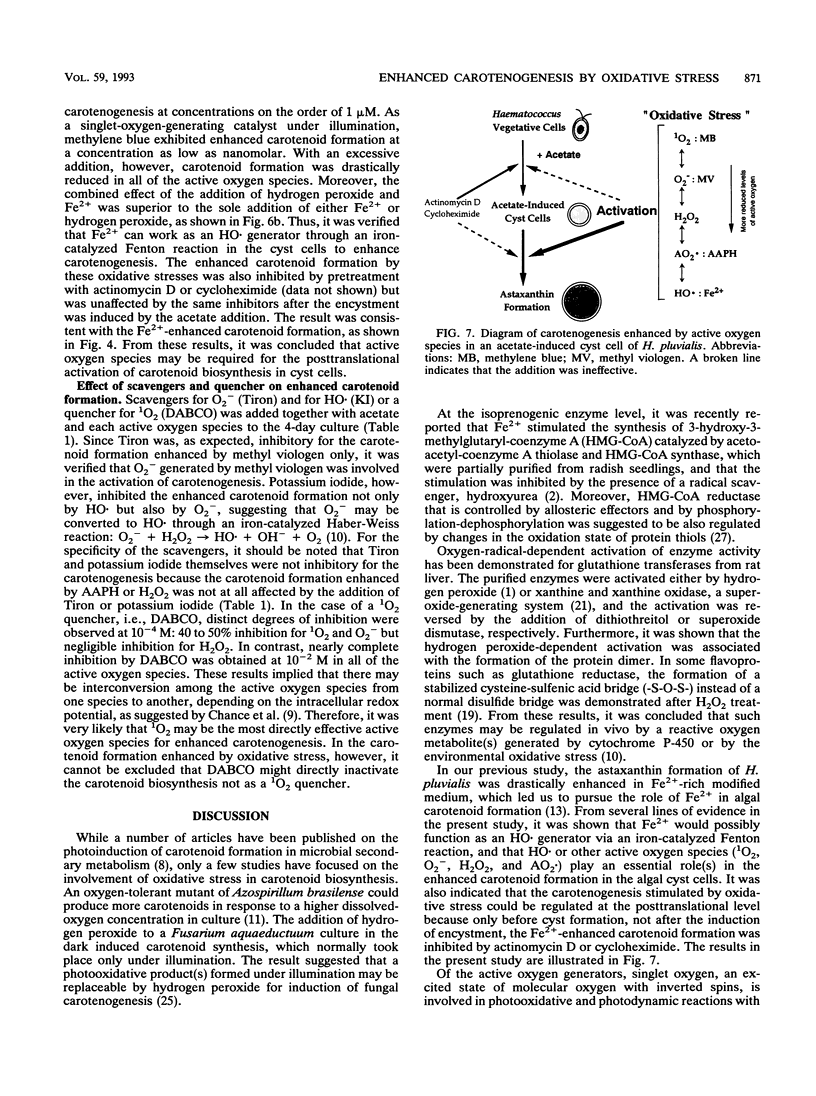
In a green alga, Haematococcus pluvialis, a morphological change of vegetative cells into cyst cells was rapidly induced by the addition of acetate or acetate plus Fe2+ to the vegetative growth phase. Accompanied by cyst formation, algal astaxanthin formation was more enhanced by the addition of acetate plus Fe2+ than by the addition of acetate alone. Encystment and enhanced carotenoid biosynthesis were inhibited by either actinomycin D or cycloheximide. However, after cyst formation was induced by the addition of acetate alone, carotenoid formation could be enhanced with the subsequent addition of Fe2+ even in the presence of the inhibitors. The Fe2+ -enhanced carotenogenesis was inhibited by potassium iodide, a scavenger for hydroxyl radical, suggesting that hydroxyl radical formed by an iron-catalyzed Fenton reaction may be required for enhanced carotenoid biosynthesis. Moreover, it was demonstrated that four active oxygen species, singlet oxygen, superoxide anion radical, hydrogen peroxide, and peroxy radical, were capable of replacing Fe2+ in its role in the enhanced carotenoid formation in the acetate-induced cyst. From these results, it was concluded that oxidative stress is involved in the posttranslational activation of carotenoid biosynthesis in acetate-induced cyst cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aniya Y., Anders M. W. Activation of rat liver microsomal glutathione S-transferase by hydrogen peroxide: role for protein-dimer formation. Arch Biochem Biophys. 1992 Aug 1;296(2):611–616. doi: 10.1016/0003-9861(92)90617-6. [DOI] [PubMed] [Google Scholar]
- Bach T. J., Boronat A., Caelles C., Ferrer A., Weber T., Wettstein A. Aspects related to mevalonate biosynthesis in plants. Lipids. 1991 Aug;26(8):637–648. doi: 10.1007/BF02536429. [DOI] [PubMed] [Google Scholar]
- Bartley G. E., Schmidhauser T. J., Yanofsky C., Scolnik P. A. Carotenoid desaturases from Rhodobacter capsulatus and Neurospora crassa are structurally and functionally conserved and contain domains homologous to flavoprotein disulfide oxidoreductases. J Biol Chem. 1990 Sep 15;265(26):16020–16024. [PubMed] [Google Scholar]
- Bartley G. E., Schmidhauser T. J., Yanofsky C., Scolnik P. A. Carotenoid desaturases from Rhodobacter capsulatus and Neurospora crassa are structurally and functionally conserved and contain domains homologous to flavoprotein disulfide oxidoreductases. J Biol Chem. 1990 Sep 15;265(26):16020–16024. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Lim B. P., Nagao A., Terao J., Tanaka K., Suzuki T., Takama K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim Biophys Acta. 1992 Jun 22;1126(2):178–184. doi: 10.1016/0005-2760(92)90288-7. [DOI] [PubMed] [Google Scholar]
- Mayer M. P., Beyer P., Kleinig H. Quinone compounds are able to replace molecular oxygen as terminal electron acceptor in phytoene desaturation in chromoplasts of Narcissus pseudonarcissus L. Eur J Biochem. 1990 Jul 31;191(2):359–363. doi: 10.1111/j.1432-1033.1990.tb19130.x. [DOI] [PubMed] [Google Scholar]
- Miller H., Claiborne A. Peroxide modification of monoalkylated glutathione reductase. Stabilization of an active-site cysteine-sulfenic acid. J Biol Chem. 1991 Oct 15;266(29):19342–19350. [PubMed] [Google Scholar]
- Murata T., Hatayama I., Satoh K., Tsuchida S., Sato K. Activation of rat glutathione transferases in class mu by active oxygen species. Biochem Biophys Res Commun. 1990 Sep 14;171(2):845–851. doi: 10.1016/0006-291x(90)91223-f. [DOI] [PubMed] [Google Scholar]
- Niki E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990;186:100–108. doi: 10.1016/0076-6879(90)86095-d. [DOI] [PubMed] [Google Scholar]
- Whitelam G. C., Codd G. A. A rapid whole-cell assay for superoxide dismutase. Anal Biochem. 1982 Mar 15;121(1):207–212. doi: 10.1016/0003-2697(82)90577-2. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]



