Abstract
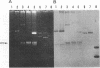
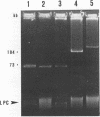
Strains of Pseudomonas syringae pv. syringae resistant to copper, streptomycin, or both compounds were recovered from symptomless and diseased tissue of four woody hosts in three nurseries in Oklahoma. In strains resistant to copper and streptomycin (Cur Smr), resistance to both compounds was cotransferred with a single plasmid which was either 68, 190, or 220 kilobase pairs (kb). All Cus Smr strains contained a 68-kb conjugative plasmid. Cur Sms strains contained one plasmid which varied in size from 60 to 73 kb. All conjugative plasmids which transferred streptomycin resistance contained sequences homologous to the strA and strB Smr genes from the broad-host-range plasmid RSF1010. The Smr determinant was subsequently cloned from a 68-kb Cur Smr plasmid designated pPSR1. A restriction map detailing the organization of the homologous Smr genes from pPSR1 and RSF1010 and cloned Smr genes from P. syringae pv. papulans and Xanthomonas campestris pv. vesicatoria revealed the conservation of all sites studied. The Cur genes cloned from P. syringae pv. tomato PT23 and X. campestris pv. vesicatoria XV10 did not hybridize to the Cur plasmids identified in the present study, indicating that copper resistance in these P. syringae pv. syringae strains may be conferred by a distinct genetic determinant.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth P. T., Grinter N. J. Comparison of the deoxyribonucleic acid molecular weights and homologies of plasmids conferring linked resistance to streptomycin and sulfonamides. J Bacteriol. 1974 Nov;120(2):618–630. doi: 10.1128/jb.120.2.618-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Cooksey D. A. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriol. 1986 Feb;165(2):534–541. doi: 10.1128/jb.165.2.534-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Cooksey D. A. Molecular cloning of copper resistance genes from Pseudomonas syringae pv. tomato. J Bacteriol. 1987 Feb;169(2):470–474. doi: 10.1128/jb.169.2.470-474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Malvick D. K., Conway K. E., George S., Pratt P. Characterization of pXV10A, a Copper Resistance Plasmid in Xanthomonas campestris pv. vesicatoria. Appl Environ Microbiol. 1990 Jan;56(1):170–175. doi: 10.1128/aem.56.1.170-175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinault A. C., Blakesley V. A., Roessler E., Willis D. G., Smith C. A., Cook R. G., Fenwick R. G., Jr Characterization of transferable plasmids from Shigella flexneri 2a that confer resistance to trimethoprim, streptomycin, and sulfonamides. Plasmid. 1986 Mar;15(2):119–131. doi: 10.1016/0147-619x(86)90048-x. [DOI] [PubMed] [Google Scholar]
- Chiou C. S., Jones A. L. Nucleotide sequence analysis of a transposon (Tn5393) carrying streptomycin resistance genes in Erwinia amylovora and other gram-negative bacteria. J Bacteriol. 1993 Feb;175(3):732–740. doi: 10.1128/jb.175.3.732-740.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey D. A. Characterization of a Copper Resistance Plasmid Conserved in Copper-Resistant Strains of Pseudomonas syringae pv. tomato. Appl Environ Microbiol. 1987 Feb;53(2):454–456. doi: 10.1128/aem.53.2.454-456.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Fukasawa K., Sakurai H., Shimizu S., Naganawa H., Kondo S., Kawabe H., Mitsuhashi S. 3''-Phosphoryldihydrostreptomycin produced by the inactivating enzyme of Erwinia carotovora. J Antibiot (Tokyo) 1980 Jan;33(1):122–123. doi: 10.7164/antibiotics.33.122. [DOI] [PubMed] [Google Scholar]
- Garde S., Bender C. L. DNA probes for detection of copper resistance genes in Xanthomonas campestris pv. vesicatoria. Appl Environ Microbiol. 1991 Aug;57(8):2435–2439. doi: 10.1128/aem.57.8.2435-2439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbride K. A., Rosendal S., Brunton J. L. Plasmid mediated antimicrobial resistance in Ontario isolates of Actinobacillus (Haemophilus) pleuropneumoniae. Can J Vet Res. 1989 Jan;53(1):38–42. [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kawabe H., Tanaka T., Mitsuhashi S. Streptomycin and Spectinomycin resistance mediated by plasmids. Antimicrob Agents Chemother. 1978 Jun;13(6):1031–1035. doi: 10.1128/aac.13.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J. M., Walker C. B. Identification of a streptomycin resistance gene and a partial Tn3 transposon coding for a beta-lactamase in a periodontal strain of Eikenella corrodens. Antimicrob Agents Chemother. 1992 Apr;36(4):740–743. doi: 10.1128/aac.36.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax A. J., Walker C. A. Plasmids related to RSF1010 from Bordetella bronchiseptica. Plasmid. 1986 May;15(3):210–216. doi: 10.1016/0147-619x(86)90039-9. [DOI] [PubMed] [Google Scholar]
- Norelli J. L., Burr T. J., Lo Cicero A. M., Gilbert M. T., Katz B. H. Homologous Streptomycin Resistance Gene Present among Diverse Gram-Negative Bacteria in New York State Apple Orchards. Appl Environ Microbiol. 1991 Feb;57(2):486–491. doi: 10.1128/aem.57.2.486-491.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintado C., Salvador C., Rotger R., Nombela C. Multiresistance plasmid from commensal Neisseria strains. Antimicrob Agents Chemother. 1985 Jan;27(1):120–124. doi: 10.1128/aac.27.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotger R., Rubio F., Nombela C. A multi-resistance plasmid isolated from commensal Neisseria species is closely related to the enterobacterial plasmid RSF1010. J Gen Microbiol. 1986 Sep;132(9):2491–2496. doi: 10.1099/00221287-132-9-2491. [DOI] [PubMed] [Google Scholar]
- Rådström P., Swedberg G. RSF1010 and a conjugative plasmid contain sulII, one of two known genes for plasmid-borne sulfonamide resistance dihydropteroate synthase. Antimicrob Agents Chemother. 1988 Nov;32(11):1684–1692. doi: 10.1128/aac.32.11.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saano A. K., Zinchenko V. V. A new IncQ plasmid R89S: properties and genetic organization. Plasmid. 1987 May;17(3):191–201. doi: 10.1016/0147-619x(87)90027-8. [DOI] [PubMed] [Google Scholar]
- Scholz P., Haring V., Wittmann-Liebold B., Ashman K., Bagdasarian M., Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989 Feb 20;75(2):271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Shareck F., Zollinger M., Sasarman A. Structure and molecular properties of the R.SmSu plasmid pSAS1206. Plasmid. 1983 Mar;9(2):215–217. doi: 10.1016/0147-619x(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Willson P. J., Deneer H. G., Potter A., Albritton W. Characterization of a streptomycin-sulfonamide resistance plasmid from Actinobacillus pleuropneumoniae. Antimicrob Agents Chemother. 1989 Feb;33(2):235–238. doi: 10.1128/aac.33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Treeck U., Schmidt F., Wiedemann B. Molecular nature of a streptomycin and sulfonamide resistance plasmid (pBP1) prevalent in clinical Escherichia coli strains and integration of an ampicillin resistance transposon (TnA). Antimicrob Agents Chemother. 1981 Mar;19(3):371–380. doi: 10.1128/aac.19.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]