Abstract
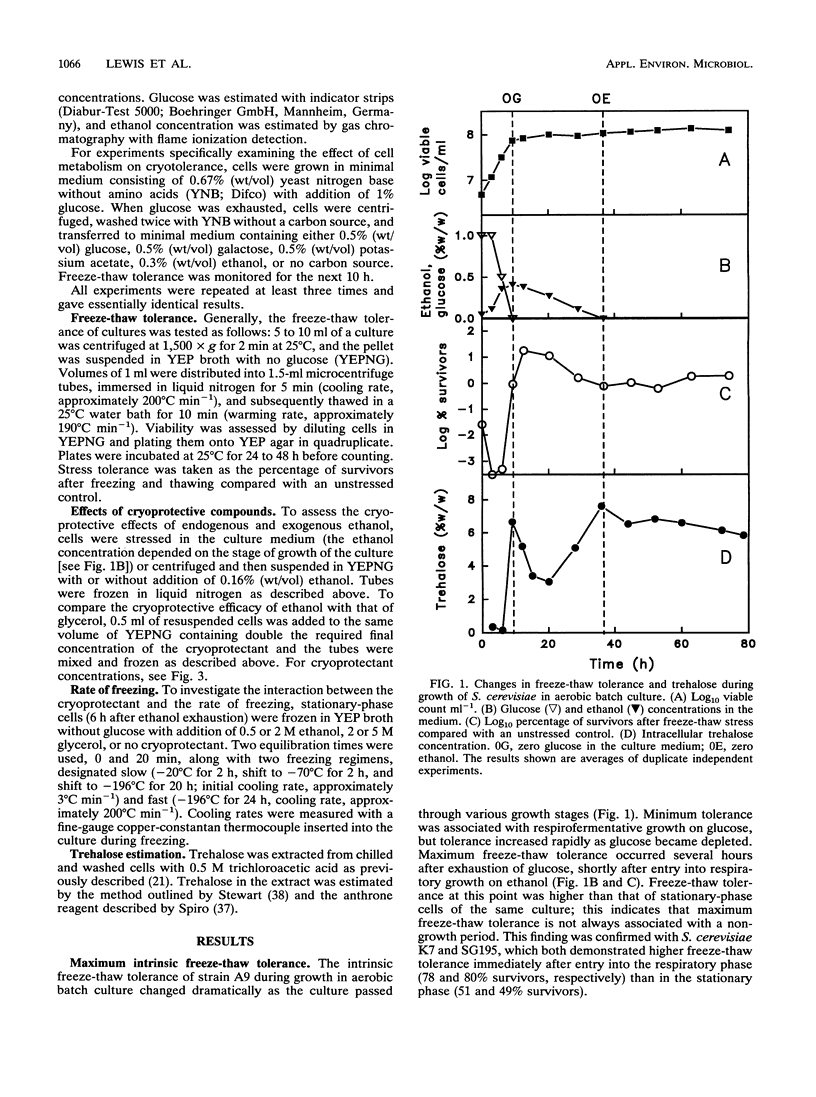
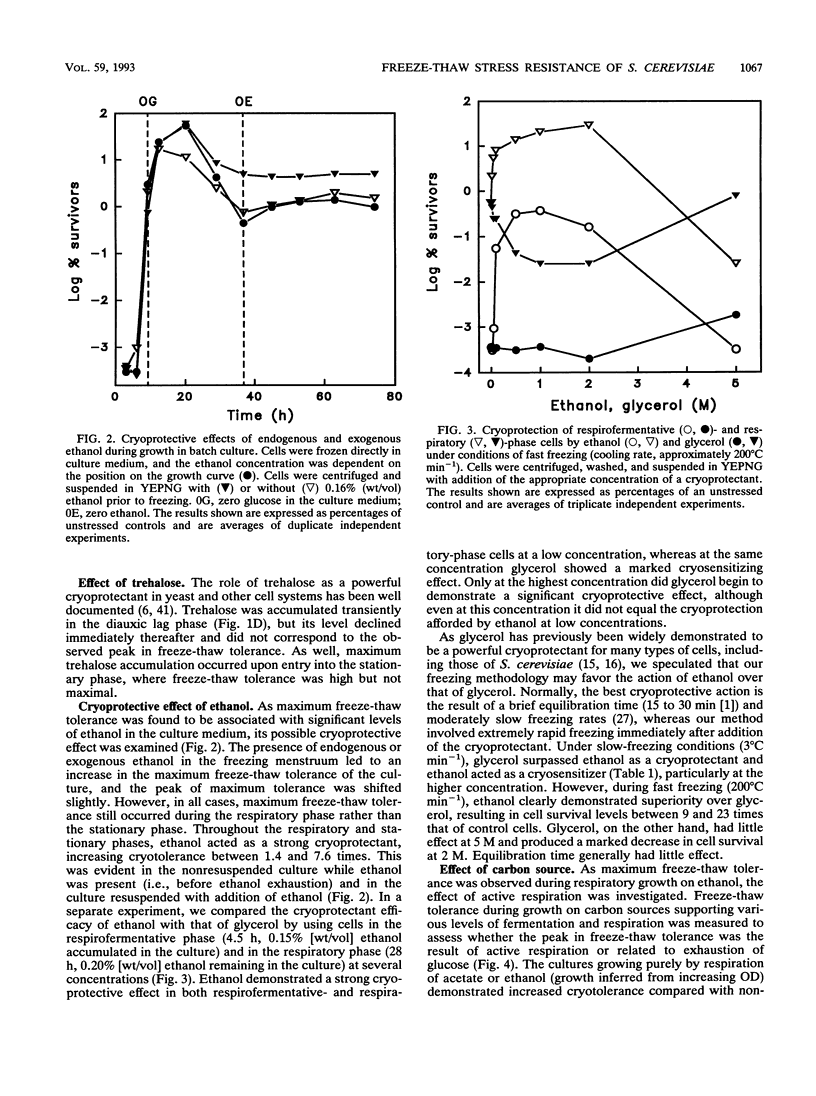
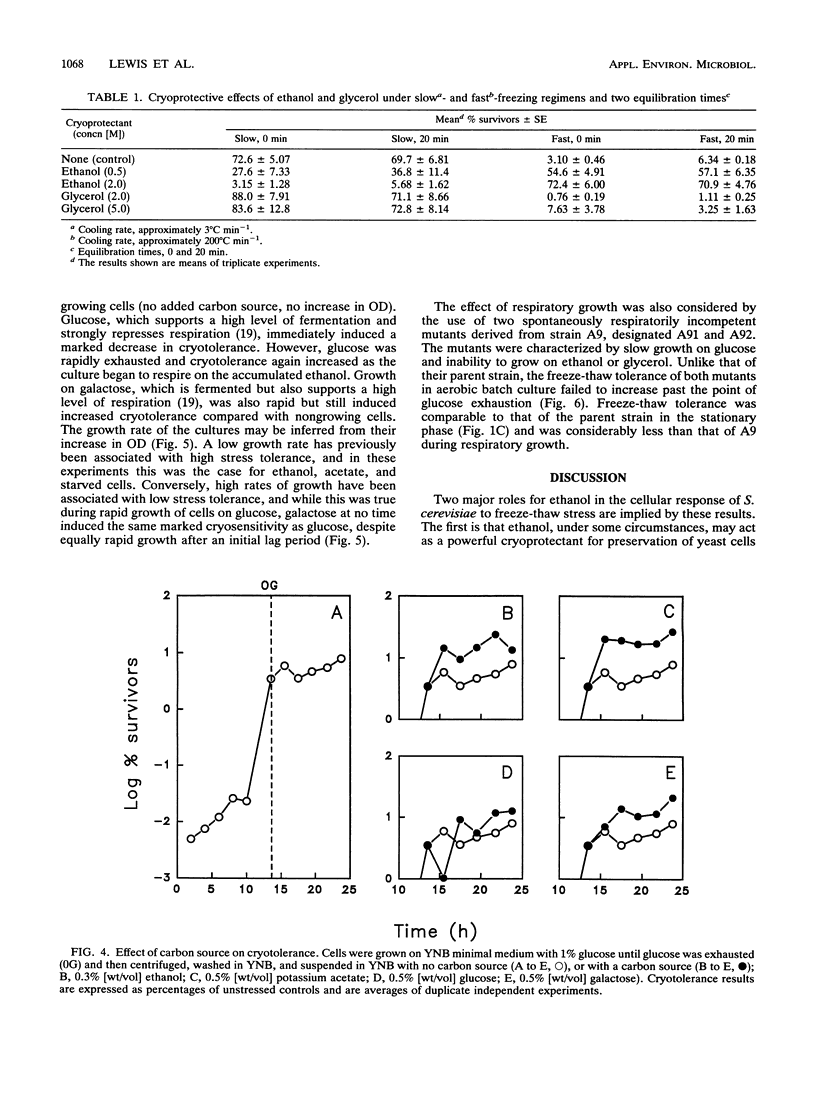
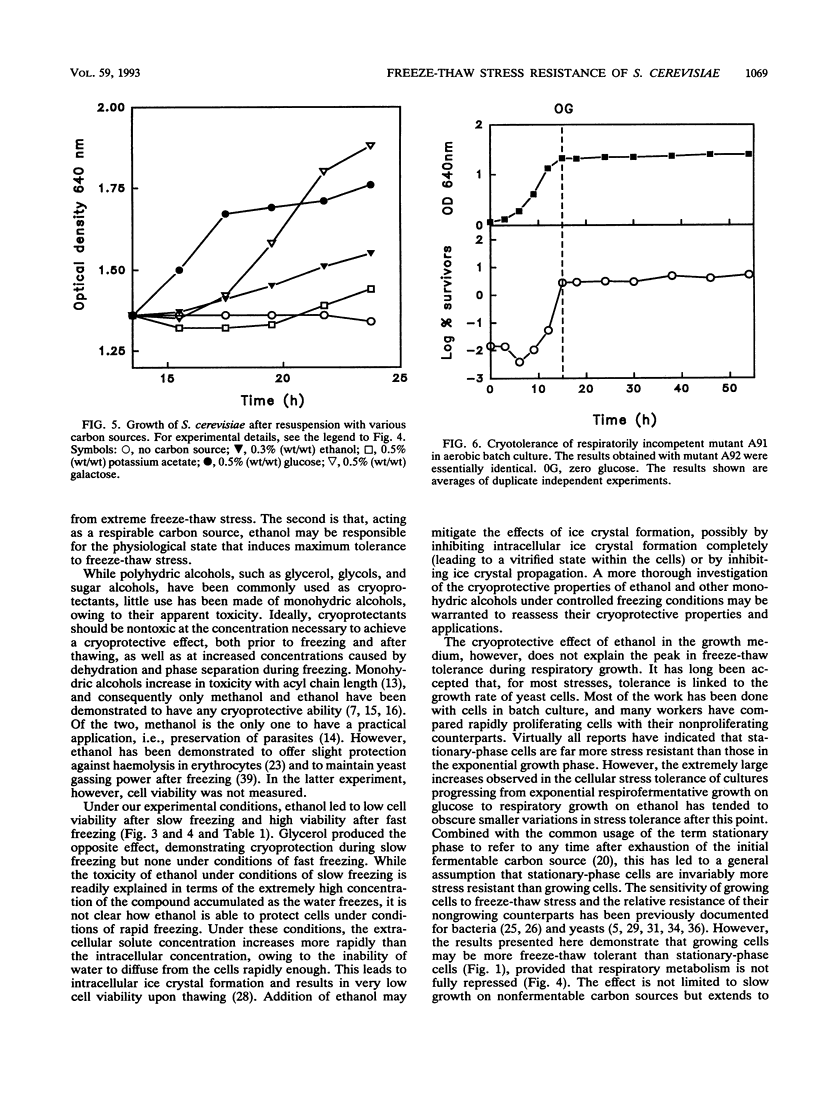
The freeze-thaw tolerance of Saccharomyces cerevisiae was examined throughout growth in aerobic batch culture. Minimum tolerance to rapid freezing (immersion in liquid nitrogen; cooling rate, approximately 200 degrees C min-1) was associated with respirofermentative (exponential) growth on glucose. However, maximum tolerance occurred not during the stationary phase but during active respiratory growth on ethanol accumulated during respirofermentative growth on glucose. The peak in tolerance occurred several hours after entry into the respiratory growth phase and did not correspond to a transient accumulation of trehalose which occurred at the point of glucose exhaustion. Substitution of ethanol with other carbon sources which permit high levels of respiration (acetate and galactose) also induced high freeze-thaw tolerance, and the peak did not occur in cells shifted directly from fermentative growth to starvation conditions or in two respiratorily incompetent mutants. These results imply a direct link with respiration, rather than exhaustion of glucose. The role of ethanol as a cryoprotectant per se was also investigated, and under conditions of rapid freezing (cooling rate, approximately 200 degrees C min-1), ethanol demonstrated a significant cryoprotective effect. Under the same freezing conditions, glycerol had little effect at high concentrations and acted as a cryosensitizer at low concentrations. Conversely, under slow-freezing conditions (step freezing at -20, -70, and then -196 degrees C; initial cooling rate, approximately 3 degrees C min-1), glycerol acted as a cryoprotectant while ethanol lost this ability. Ethanol may thus have two effects on the cryotolerance of baker's yeast, as a respirable carbon source and as a cryoprotectant under rapid-freezing conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bataillé N., Thoraval D., Boucherie H. Two-dimensional gel analysis of yeast proteins: application to the study of changes in the levels of major polypeptides of Saccharomyces cerevisiae depending on the fermentable or nonfermentable nature of the carbon source. Electrophoresis. 1988 Nov;9(11):774–780. doi: 10.1002/elps.1150091113. [DOI] [PubMed] [Google Scholar]
- Boucherie H. Protein synthesis during transition and stationary phases under glucose limitation in Saccharomyces cerevisiae. J Bacteriol. 1985 Jan;161(1):385–392. doi: 10.1128/jb.161.1.385-392.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell S. F. Yeast freeze--thaw survival rates as a function of different stages in the cell cycle. Cryobiology. 1981 Oct;18(5):506–510. doi: 10.1016/0011-2240(81)90210-8. [DOI] [PubMed] [Google Scholar]
- Frim J., Kruuv J., Frey H. E., Raaphorst G. P. Survival of unprotected, mammalian plateau-phase cells following freezing in liquid nitrogen. Cryobiology. 1976 Aug;13(4):475–483. doi: 10.1016/0011-2240(76)90104-8. [DOI] [PubMed] [Google Scholar]
- Gélinas P., Fiset G., Leduy A., Goulet J. Effect of growth conditions and trehalose content on cryotolerance of bakers' yeast in frozen doughs. Appl Environ Microbiol. 1989 Oct;55(10):2453–2459. doi: 10.1128/aem.55.10.2453-2459.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas P., Fiset G., Willemot C., Goulet J. Lipid content and cryotolerance of bakers' yeast in frozen doughs. Appl Environ Microbiol. 1991 Feb;57(2):463–468. doi: 10.1128/aem.57.2.463-468.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino A., Mihara K., Nakashima K., Takano H. Trehalose levels and survival ratio of freeze-tolerant versus freeze-sensitive yeasts. Appl Environ Microbiol. 1990 May;56(5):1386–1391. doi: 10.1128/aem.56.5.1386-1391.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Buttke T. M. Effects of alcohols on micro-organisms. Adv Microb Physiol. 1984;25:253–300. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- Karow A. M., Jr Cryoprotectants--a new class of drugs. J Pharm Pharmacol. 1969 Apr;21(4):209–223. doi: 10.1111/j.2042-7158.1969.tb08235.x. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Kaul S. C., Iwahashi H., Obuchi K. Do heat shock proteins provide protection against freezing? FEMS Microbiol Lett. 1990 Oct;60(1-2):159–162. doi: 10.1016/0378-1097(90)90364-v. [DOI] [PubMed] [Google Scholar]
- Kruuv J., Lepock J. R., Keith A. D. The effect of fluidity of membrane lipids on freeze-thaw survival of yeast. Cryobiology. 1978 Feb;15(1):73–79. doi: 10.1016/0011-2240(78)90009-3. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E. The protective action of neutral solutes against haemolysis by freezing and thawing. Biochem J. 1954 Feb;56(2):265–270. doi: 10.1042/bj0560265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas R. Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast. 1986 Dec;2(4):221–228. doi: 10.1002/yea.320020403. [DOI] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie K. F., Singh K. K., Brown A. D. Water stress plating hypersensitivity of yeasts: protective role of trehalose in Saccharomyces cerevisiae. J Gen Microbiol. 1988 Jun;134(6):1661–1666. doi: 10.1099/00221287-134-6-1661. [DOI] [PubMed] [Google Scholar]
- Mackey B. M. Lethal and sublethal effects of refrigeration, freezing and freeze-drying on micro-organisms. Soc Appl Bacteriol Symp Ser. 1984;(12):45–75. [PubMed] [Google Scholar]
- Mazur P. Cryobiology: the freezing of biological systems. Science. 1970 May 22;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- Meryman H. T. Cryoprotective agents. Cryobiology. 1971 Apr;8(2):173–183. doi: 10.1016/0011-2240(71)90024-1. [DOI] [PubMed] [Google Scholar]
- Meyer E. D., Sinclair N. A., Nagy B. Comparison of the survival and metabolic activity of psychrophilic and mesophilic yeasts subjected to freeze-thaw stress. Appl Microbiol. 1975 Jun;29(6):739–744. doi: 10.1128/am.29.6.739-744.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel R. E., Morrison D. P. Heat-shock induction of ionizing radiation resistance in Saccharomyces cerevisiae, and correlation with stationary growth phase. Radiat Res. 1982 May;90(2):284–291. [PubMed] [Google Scholar]
- Parry J. M., Davies P. J., Evans W. E. The effects of "cell age" upon the lethal effects of physical and chemical mutagens in the yeast, Saccharomyces cerevisiae. Mol Gen Genet. 1976 Jul 5;146(1):27–35. doi: 10.1007/BF00267979. [DOI] [PubMed] [Google Scholar]
- ROSENBERG A. M., WOOD T. H. The modifying effect of culture age on heat sensitivity of yeast. Exp Cell Res. 1957 Jun;12(3):692–694. doi: 10.1016/0014-4827(57)90193-3. [DOI] [PubMed] [Google Scholar]
- SHERMAN F. The heat inactivation and production of cytochrome deficiency in yeast. Exp Cell Res. 1956 Dec;11(3):659–660. doi: 10.1016/0014-4827(56)90180-x. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Taulien J., Borkovich K. A., Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992 Jun;11(6):2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasima T., Yasukawa M. Dependence of freeze--thaw damage on growth phase and cell cycle of cultured mammalian cells. Cryobiology. 1977 Jun;14(3):379–381. doi: 10.1016/0011-2240(77)90186-9. [DOI] [PubMed] [Google Scholar]


