Abstract
The incidence of hepatocellular carcinoma (HCC) shows marked variation worldwide but the magnitude of this tumor is reflected by the occurrence of at least 1 million new cases annually and the uniformly dismal outlook with median survivals of <25 months after resection and <6 months with symptomatic treatment. The strikingly uneven distribution of this tumor parallels the prevalence of hepatitis B infection with rising incidence in western countries attributed to hepatitis C infection. Chronic hepatitis and cirrhosis constitute the major preneoplastic conditions in the majority of HCCs and may be related to other etiologic agents such as environmental chemical carcinogens including nitrites, hydrocarbons, solvents, organochlorine pesticides, and the chemicals in processed foods, cleaning agents, cosmetics and pharmaceuticals, as well as plant toxins such as anatoxins produced by fungi that cause spoilage of grain and food in the tropics. Genetic diseases such as genetic hematochromatosis, Wilson's disease, α-1-antitrypsin deficiency, and the inborn errors of metabolism including hereditary tyrosinemia and hepatic porphyria, are known to be associated with HCC. Numerous genetic alterations and the modulation of DNA methylation are recognized in HCC and it is likely that these genetic and epigenetic changes combine with factors involved in chronic hepatocyte destruction and regeneration to result in neoplastic growth and multiple molecular pathways may be involved in the production of subsets of hepatocellular tumors.
Keywords: Hepatocellular carcinoma, pathogenesis, epidemiology, hepatitis, alcohol, cirrhosis, risk factors, carcinogens
Introduction
Hepatocellular carcinoma (HCC) is one of the most common internal malignancies worldwide. In some countries of high incidence, HCC is the leading form of cancer and overall it rates as the seventh most common malignancy in males and the ninth in females 1,2,3,4.
Cancer statistics from many of the countries with a high incidence of HCC are incomplete; as such much of the data available may represent under-estimates 5. At least 1 million new cases of HCC occur annually and mortality from the disease remains high despite treatment 2,3,4, with recent results showing 1-year, 3-year, and 5-year survival rates of 66.1%, 39.7%, and 32.5% respectively, overall and 93.5%, 70.1% and 59.1%, respectively, for early stage patients 6. Even in countries where the incidence is low, median survival time after resection is 24.8 months compared with 5.8 months in symptomatically treated patients 7.
Geographic distribution
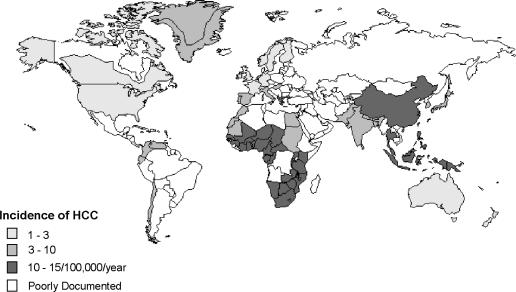
The geographic distribution of HCC worldwide is strikingly uneven (Figure 1). South-east Asian countries (Taiwan, Korea, Thailand, Hong Kong, Singapore, Malaysia, southern China) and tropical Africa show the highest incidence, in the region of 10–20 per 100 000 population. The prevalence rates vary again among these countries, with an incidence of 150 per 100 000 in Taiwan 2 and 28 per 100 000 population in Singapore 8. Similarly high incidence rates are suspected in Cambodia, Vietnam, and Burma but accurate documentation is lacking. The lowest rates of 1–3 per 100 000 population for HCC are found in western countries, Australia, South America, and India 9, with intermediate rates in Japan, the Middle East, and Mediterranean countries 2,3,4,10,11.
Figure 1. .
Incidence of hepatocellular carcinoma worldwide.
In general, the incidence of HCC in migrant populations slowly equates to that of the local population with successive generations. Indians who have settled in Hong Kong and Singapore have acquired incidence rates close to those of the rest of the population and about double that of their home country; whereas the incidence among Japanese and Korean migrants in California and Hawaii has slowly decreased. The exceptions appear to be Chinese populations, who seem to be at high risk regardless of location whether it be Singapore, Shanghai, Hong Kong or elsewhere, and Whites, who retain a low incidence even when living in areas of high prevalence such as South-east Asia or Africa. This maintenance of risk has been attributed to the continuance of the lifestyle and environment of their home countries and parallels the hepatitis B virus (HBV) carrier rates in these populations 2,4,11,12.
This remarkable geographical distribution has prompted investigation into location-specific etiological factors. It is unlikely that HCC results from a single causative agent. As with other carcinomas, a multi-step mechanism involving complex interactions between multiple etiological factors is more probable. Race and genetic factors have been found to be of no etiological significance, rather, environmental agents are closely related, in particular, the prevalence of chronic HBV infection. Hepatitis C virus (HCV) infection is also emerging as a major etiological factor, with increasing rates of HCV infection thought to partly underlie the increasing incidence of HCC in the western world 13. The majority of HCCs arise in the setting of chronic hepatitis and cirrhosis. Carcinogenesis of hepatocytes represents a linear and progressive process in which successively more aberrant monoclonal populations of liver cells evolve. Regenerative hepatocytes in focal lesions in the inflamed liver give rise to hyperplastic nodules that progress to dysplastic nodules that are thought to be the direct precursor of HCC. The neoplastic transformation often results from the accumulation of genetic changes during the repetitive cellular proliferation that occurs in the damaged liver.
Risk factors
Lesser variations in the incidence of HCC have been observed in racially homogeneous countries such as Greece, Spain, and Italy. Such differences have been explained by differences in HBV carriage, alcohol consumption and smoking, or variations in exposure to hepatotoxins. Switzerland, for example, a highly developed and industrialized country, has a higher than average rate of HCC compared with other European nations, raising the possibility of additional risks such as exposure to hepatotoxic chemicals. In an astounding survey of 840 million people in China during 1972–1977, it was found that the main endemic areas for HCC were along the south-east coast, particularly the deltas, valleys, and islands. In these areas the standardized mortality rate from HCC was >60 per 100 000 people per year compared with <6 per 100 000 people in low-incidence areas of the country 14. In Mozambique, a ninefold difference between the coastal and inland region has been reported. Movement from a rural to an urban environment has also been associated with increased risk in countries like Norway and Poland, whereas the reverse seems to be true for South Africa 2,3,4. Differences in levels of exposure to environmental hepatotoxins and improvements in living standards are thought to be responsible for these differences.
In China, high mortality rates from HCC have been reported in coastal and riverside areas with stagnant and polluted water supplies. However, improved living standards can produce paradoxical effects. While they may reduce the incidence of HCC in some communities, studies on time trends show a steady but indisputable rise in liver cancer rates. In Japan, the rate of HCC has risen from 1.91% among 19 357 autopsies in 1958–1959 to 7.66% in 1986–1987 5. A similar rise was observed in Los Angeles where the rate rose from 0.15% in 1918–1953 to 1.48% in 1964–1983 15. There seems to be a general increase in incidence of liver cancer throughout the world with reported increases among males and females in 24 and 26 of 37 countries, respectively, whose cancer registries were examined 16. Florence, Italy, reported an eightfold increase, Shanghai a twofold increase between 1959 and 1976 and Mexico a twofold increase over a 25-year period 17. It is unlikely that HCC is due to a single causative agent; more likely, as with other carcinomas, this tumor is the result of a complex interaction between multiple etiological factors and through a multi-step mechanism. The risk factors for HCC may be divided into genetic, and environmental and biological factors, the more common of these are discussed below.
Age and gender
HCC may occur from as early as 2 years of age in areas of high incidence 18. In general, the incidence increases with age in all populations and shows a slight decrease in the elderly. The age peak in a given region tends to be inversely related to the frequency of the tumour, i.e. the age peak is in younger patients in areas of high incidence, and in elderly patients in areas of low incidence. In Mozambique, where 50% of patients with HCC are <30 years old, the incidence of the tumor among males aged 25–34 years is >500-fold that of the same age group in low prevalence western countries. This compares with only a 15-fold difference between the elderly of both populations. Recent increases in incidence in countries such as the USA have been accompanied by a shift to a younger average patient age 13.
HCC shows a strong male predilection, being four and eight times more common in males in low and high prevalence regions, respectively. While this finding may be partly attributed to the cumulative result of other associated factors such as the higher incidence of cirrhosis in males, and higher levels of smoking and alcohol intake, findings in experimental animals suggest that sex hormones and/or hormone receptors may play a role. Orchidectomy reduces the carcinogenic effects of chemicals in male rats to the level found in females. Implantation of stilbesterol or estradiol pellets produces a similar but less marked effect 19,20. Most liver cancers show elevation of androgen receptors 21,22, although the results of treatments targeting hormone action and receptors have produced variable or disappointing results 23,24. The rate of DNA synthesis in cirrhotic livers, a factor related to the risk of carcinoma in such livers, is higher in men than in women 25. Liver adenomas associated with androgen or anabolic steroids may regress with withdrawal of the drug 26, a phenomenon also seen in tumors induced by oral contraceptive steroids. Sex steroids most likely act in combination with other factors as promoters of abnormal growth.
HCC occurs in adolescence and childhood and has been reported in children as young as 2 years of age in Hong Kong 18. This is not unexpected in high incidence populations where the tumor is associated with HBV infection contracted early in life 27. Congenital abnormalities and inborn errors of metabolism may account for some cases, especially in western countries 28. Other tumors including hepatoblastoma and fibrolamellar carcinoma have a predilection for the young 29.
Genetic and congenital abnormalities
While a genetic susceptibility to cirrhosis and liver cancer has been demonstrated in inbred strains of mice, the same has not been established in man. Familial clustering of HCC has been described in Chinese and Alaskan natives 30,31 and cases of liver cancer have been recorded in children of several families for up to three generations 32,33, but these have invariably been associated with chronic HBV infection, as shown in the majority of cases. Analysis of major histocompatibility complex antigens among patients and controls in both South Africa and China has not revealed a link with HBV infection or liver cancer.
Rarely, liver cancer occurs in association with conditions that have a genetic, congenital or metabolic origin. HCC has been rarely documented in familial polyposis coli 34, ataxia telangiectasia 35, familial cholestatic cirrhosis, congenital hepatic fibrosis, neurofibromatosis, situs inversus and the fetal alcohol syndrome 29,36.
Among the inborn errors of metabolism, the chronic form of hereditary tyrosinemia carries the highest risk of liver malignancy, with one report describing liver cancer in 16 of 43 patients 37. Such patients showed a rapid progression from micro- to macro-nodular cirrhosis within a period of a few months, and then to dysplasia and eventually HCC. To avert the latter complication, hepatectomy and liver transplantation before 2 years of age is now the recommended treatment in this condition 38. Type I glycogen storage disease may be associated with adenomas but carcinoma has rarely been reported. Hepatic porphyria of both intermittent and cutanea tarda types have a 61-fold increased risk for HCC 39.
In one study of genetic hemochromatosis, 22% of patients died with HCC, representing a 219-fold increase over the general population 40. Males were commonly affected with cirrhosis and its attendant risk of liver cancer. Iron has been suggested to have carcinogenic properties through the production of free radicals but this has not been substantiated. Wilson's disease, another autosomal recessive disorder, also affects males more frequently and produces cirrhosis through the accumulation of copper in hepatocytes. A few cases of HCC have been reported in this disorder but always accompanied by cirrhosis. Rarely, HCC has complicated biliary cirrhosis, another condition in which excess of copper accumulates in the liver.
Alpha-1-antitrypsin (αlAT) deficiency is associated with jaundice and cirrhosis in early childhood, and with pulmonary emphysema and cirrhosis in adult life. The enzyme is synthesized in the liver and released into the blood. It is an inhibitor of serine proteinases, which include trypsin, chymotrypsin and leukocyte elastase. In αlAT deficiency, the enzyme continues to be produced in the liver but is not secreted, accumulating as visible globules in the hepatocytes. Up to 75 allelic variants of the Pi (protease inhibitor) genes control this enzyme. PiZ is the variant associated with low levels of serum αlAT and occurs as a homozygous but more commonly as a heterozygous form. The mechanisms behind the occurrence of a α1AT deficiency and HCC are still not known. αlAT globules can also be seen in the tumor cells of both adenomas and carcinomas of patients who do not have the PiZ gene and who show no evidence of αlAT deficiency, suggesting that the failure to release the enzyme may have a promoting effect in carcinogenesis by allowing local proteases to destroy contact inhibition that otherwise occurs between transformed liver cells 41. However, it is likely that other factors may also be operative, as the association with HCC appears to be statistically significant only for males.
Membranous obstruction of the hepatic portion of the inferior vena cava, a type of Budd-Chiari syndrome, has been associated with HCC. This condition is uncommon in the West and is seen in Japan, India and among the black population of South Africa. The lesion may be congenital, such as due to malformation of the Eustachian valve, or acquired due to mechanical injury, infection or thrombosis. In Japan, 29 of 71 cases (41%) developed HCC, and in South Africa 20% of all cases with HCC showed the lesion at autopsy and 47.5% of patients with radiologically demonstrated caval obstructions developed HCC 42. In this condition passive congestion may act as a stimulus to hepatocytes’ regeneration, although the true mechanism leading to carcinoma is not known.
Cirrhosis
Cirrhosis is the most common association of HCC, being the underlying disease in 80–90% of patients with primary liver cancer in most countries. Nonalcoholic post-hepatitic cirrhosis is the most common association, but any condition that causes cirrhosis may potentially lead to HCC, including conditions such as inborn errors of metabolism, hereditary hemochromatosis, αlAT deficiency, and Wilson's disease 37,40,43,44,45.
In a rare strain of rat in which severe hepatic necrosis occurs spontaneously, survivors invariably develop liver cancer after a period of chronic liver disease. Almost any form of chronic liver disease that leads to cirrhosis may be complicated by HCC, and cirrhosis, whatever the cause, is a precancerous condition.
It has been shown that cirrhotic livers with large nodules and thin intervening stroma are more commonly associated with HCC than livers with small nodules and thick stroma 46. Larger nodules are thought to have greater regenerative activities, with increased DNA synthesis in hepatocytes, more rearrangements of DNA sequences and hence greater vulnerability to mutagenesis following exposure to another co-factor. In patients with alcoholic cirrhosis, a higher incidence of carcinoma was noted among those who had abstained and whose micronodular cirrhosis had turned macronodular, perhaps similarly linked to the surge of regenerative activity that transforms small nodules to large ones. Clinically, patients with alcoholic cirrhosis seldom develop carcinoma while they are still imbibing.
Cirrhosis is clearly not a prerequisite for HCC and the latter is not an inevitable consequence. The two conditions share a common cause, with some causes of cirrhosis, such as chronic HBV infection, being associated with a higher risk of HCC than others, such as alcohol.
Hepatitis B virus (HBV)
An etiological association between HBV and HCC has been clearly established, although the relationship is complex and involves other etiologic factors. Eighty per cent of cases of HCC worldwide are estimated to be etiologically associated with HBV infection 47 and the incidence of HCC parallels carrier rates of HBV infection. Improved control of HBV infection from universal vaccination has resulted in a recent decline in HCC in countries such as Taiwan and China 48,49.
Chronic infection with HBV imparts a 200-fold increased risk of developing HCC. Acquisition of HBV infection at birth or in early childhood is associated with a greatest risk of becoming a carrier and subsequently developing HCC. This is attributed to the immaturity of the immune system in this age group. The risk falls with increasing age, with about 40% if infected in childhood and 10% risk of carrier state if infected as an adult 50,51. Familial clustering of HCC is commonly due to HBV-related disease as a result of vertical transmission of the virus.
Carcinogenesis is thought to result from the chronic hepatitis and cirrhosis caused by HBV, as well as from viral integration. While HBV antigens can readily be demonstrated by immunostaining in the non-tumorous hepatocytes of carriers and patients with cirrhosis and HCC, they are less commonly found in the tumor cells. HBV cannot be visualized in tumor cells in a replicative form, but it can be demonstrated once integrated, by molecular techniques. Integration of HBV DNA into the host genome always precedes development of HCC, although the site of integration is random 52,53. The precise effects of integration are yet to be determined. It may result in transactivation of proto-oncogenes, activation of growth factors, and inactivation of tumor suppressor genes leading to abnormal cell growth. The HBx gene encoded by HBV may also contribute to the development of HCC through a variety of effects on multiple systems including cyclin A, protein kinases, and DNA repair. When HBV DNA was used as a genetic marker, identical patterns of integration were found in multifocal hepatomas, and in primary tumors and their metastases, indicating an origin from a single clone of cells in which HBV integration had occurred before malignant transformation 52,53.
Hepatitis C virus (HCV)
Hepatitis C virus is now emerging as the leading cause of HCC in western countries. HCC rates in the United States have increased by 70% over the last two decades, with similar trends reported in Canada and Western Europe 47. While some of the documented increase may be artefactual and a result of greater availability of specialist medical services and hence increased reporting of cases 54, at least half of this increase in the USA has resulted from HCV-related cases. Chronic infection by HCV is a leading risk factor in non-Asians 55,56. The HCV carrier rate among Japanese blood donors is 1.2% and may be lower in western countries. Antibodies to HCV have been found in as high as 76% of patients with HCC in Japan, Italy, and Spain 4.
HCV causes chronic liver disease, with eventual development of cirrhosis and HCC. Unlike HBV, HCV is a single-stranded RNA virus that does not integrate into the host genome. There is currently no evidence that HCV is of itself oncogenic; however, HCC may rarely develop in non-cirrhotic HCV-infected individuals, so a direct oncogenic effect cannot be excluded.
Interestingly, there are suggestions that the presence of HBV gene in patients with chronic HCV-associated liver injury appears to promote hepatocarcinogenesis 57,58, but this requires further confirmation. Human immunodeficiency virus (HIV) co-infection results in greater likelihood of chronicity and enhanced viral replication in both HBV and HCV infections. HIV co-infection hastens HCV-related liver disease with faster progression to cirrhosis, end-stage liver disease and the occurrence of HCC. In contrast, current evidence suggests that HIV infection may have a negative impact on HBV-related liver disease progression, although the mechanisms for this are unclear 59.
Other hepatitis viruses
Other hepatitis viruses have an uncertain role in hepatocarcinogenesis. There is an obligatory symbiosis between hepatitis D virus (HDV) and HBV, making evaluation of its role in hepatocarcinogenesis difficult. However, there is evidence to indicate that HDV infection places additional burden on the already damaged liver, thus contributing to the risk of carcinoma. Hepatitis A and hepatitis E infections do not lead to chronic liver disease and have no carcinogenic role.
Plant carcinogens
Large doses of afiatoxins produced by the fungi Aspergillus flavis and A. parasitans are well recognized to cause severe hepatic injury. These fungi grow readily on grains, peanuts, and food products in the humid subtropical and tropical regions, and A. flavis is the most common cause of food spoilage in the tropics.
Regions where afiatoxin intake is common also tend to have high levels of HBV infection, making epidemiologic analysis difficult, but it appears that chronic exposure to afiatoxin is carcinogenic. Chronic feeding of afiatoxin Bl, the most hepatotoxic of the afiatoxins, induced liver cancer in many animal species. The intake of afiatoxin Bl by inhabitants of 10 villages in China was shown to correlate with HCC mortality rates 60.
Higher HCC mortality rates have also been found in people who drink pond-ditch water contaminated with the blue-green algal toxin microcystin, the toxin also causing hepatic hemorrhage and necrosis 60. Other mycotoxins such as sterigmatocystin, produced by Aspergillus, and luteoskyrin and cyclochlorotine, metabolites of Penicillium islandicum found in spoilt rice and grain, have been demonstrated to have carcinogenic effects in experimental animals, but similar effects have not been established in humans.
Chemical carcinogens
Variations in HCC incidence rates within a region may also be explained by differences in levels of exposure to chemical carcinogens. Improved living conditions can result in the increased use of a wide variety of chemicals in industry and in items such as processed foods, cleaning reagents, cosmetics, and pharmaceuticals. Other chemicals like nitrites, hydrocarbons, solvents, organochlorine pesticides, primary metals, and polychlorinated biphenyls have also been implicated as potential carcinogens. Many of these are hepatotoxic and have been shown experimentally to have carcinogenic potential. Sweden, a highly developed and industrialized country, has a higher HCC rate than other European nations. Chinese farmers from Qidong province who drank ditch water contaminated with pesticides such as DDT, which was once widely used, were found to have a crude death rate from HCC of 62–110 per 100 000 population, compared with 0–11.9 deaths per 100 000 among those who drank well-water. The sinking of more wells in the country resulted in a 20–30% reduction in the frequency of liver cancer 61.
Radiation and Thorotrast
The victims of the Hiroshima and Nagasaki atomic bombing did not show evidence of increased liver cancer, although there is good evidence that internal α and β radiation is carcinogenic. Thorotrast, colloidal thorium dioxide, used as an angiographic agent in the 1930s, emits high levels of α, β and γ radiation with a long physical and biological half-life. Thorotrast accumulates in the macrophages of the reticuloendothelial system, particularly the liver, and produces hepatic fibrosis, angiosarcoma, cholangiocarcinoma, and HCC. Angiosacoma was more commonly associated with Thorotrast in western countries while in Japan, both cholangiocarcinoma and HCC were more common. HCC developed at least 10 years after the deposition of Thorotrast in the liver compared with shorter intervals required for the other two tumors.
Miscellaneous factors
Malnutrition is common in many of the geographic areas of high prevalence of HCC but the association is more likely due to HBV infection and hepatotoxins that are also prevalent in these areas. Existing information suggests that over-nourishment is more likely to promote neoplastic growth, as shown by the association of a high intake of animal fat and cholesterol, and obesity, with cancer of the breast, endometrium, colon, and pancreas 62. Prolonged parenteral nutrition in infancy may be complicated by cholestasis, liver fibrosis and cirrhosis, with rare cases of liver cancer 63.
There is no evidence to link parasitic infections with HCC, although the relationship between liver flukes and cholangiocarcinoma is well recognized. It is possible that certain types of medication may expedite hepatocarcinogenesis. Anecdotal case reports have incriminated azathioprine, methotrexate, denazol, tamoxifen, and cytoproterone acetate in this role. There are also very rare reports of HCC developing in various forms of chronic liver disease including autoimmune chronic hepatitis and primary biliary cirrhosis.
Chronic alcohol abuse often complicates HCC, especially in low-incidence areas where HBV infection is uncommon. While alcohol has been incriminated in the causation of carcinomas in the larynx, mouth, and esophagus, it is not a cirrhosis-causing agent and has not been shown to have a carcinogenic effect in the liver. Alcohol may have a role as a co-carcinogen with other agents such as HBV, HCV, hepatotoxins, and tobacco. Alcohol may also have a role through its induction of the microsomal cytochrome P450 system, which is responsible for the metabolic activation and inactivation of diverse chemical carcinogens including aflatoxins. The cytochrome P450 system is also highly inducible by smoking, which is a significant risk factor for HCC, having also a synergistic effect with alcohol and chronic HBV infection 64.
While a vast array of naturally occurring substances, which can be found in drinking water, foodstuffs, native and herbal remedies, have been suspected carcinogens; most have not been proven to be so. Among these substances are the pyrrolizidine alkaloids found in species of Senecio, Crotalaria and Heliotropium plants, comfrey, which is used as a green vegetable, tea and animal fodder, and cycads that contain the glycoside cycasins have been shown to be hepatotoxic and can produce liver tumors in many animals. Other substances like tannic acid in tea and coffee, and safrole in oils used for medicines and flavoring, are carcinogenic in rodents.
Precancerous changes and hepatocarcinogenesis
The concept of premalignant lesions of the liver and cellular alterations preceding fully developed HCC has been controversial. Recent refinements in imaging allow the identification and resection of nodular lesions of <1 cm diameter and liver transplantation occasionally provides explanted liver tissues with early or premalignant lesions for more exacting morphological and molecular examination.
The diagnostic criteria for early HCC include nuclear crowding, increased cytoplasmic basophilia, and microacinar formation 65. These criteria have been successfully employed for evaluating ultrasound-guided needle biopsies of nodular hepatic lesions 66. Tumor size is another important criterion, as one study involving 58 resected small nodular lesions revealed every lesion exceeding 1.5 cm diameter to be an early carcinoma 67. However, the sizes of benign and early malignant lesions may overlap and adenomas can exceed 2 cm in diameter. The liver cell populations that precede the development of overt metastasizing HCC are characterized by hyperplastic expansive collections of hepatocytes, which may have clear, basophilic or acidophilic cytoplasm or may show pleomorphism and megalocytosis. The gradual loss of adult liver enzymes and the appearance of fetal enzymes accompany these features. Such changes are recognized as dysplastic nodules (adenomatous hyperplasia) and liver cell dysplasia.
Dysplastic nodule
The dysplastic nodule, also known as adenomatous hyperplasia, hepatocellular pseudotumor, macroregenerative nodule, adenomatous regeneration, adenomatous hyperplastic nodule, and borderline nodule, is defined as a nodule that is usually, but not always, found in the setting of cirrhosis and is distinct from the surrounding parenchyma in terms of size, color, texture, and bulging cut surface 68. These nodules grow to a large size and appear tumor-like, ranging from 1 to 15 cm, most measuring 2–3 cm. Such nodules are readily detected by modern imaging techniques and occur usually in individuals who are being screened for HCC. They may occur in the presence of established HCC and are often larger and more numerous in this situation. The term “dysplastic nodule” does not refer to a nodule made up of dysplastic hepatocytes of small or large cell type, in fact, they often consist of normal-appearing hepatocytes with only foci of cellular atypia within the nodules. They are not encapsulated but are often partially or completely surrounded by a rim of fibrous tissue in the manner of ordinary cirrhotic nodules. The nodules have a bulging cut surface and show compression of the surrounding liver parenchyma. Unlike focal nodular hyperplasia, they do not show a central scar and do not contain necrotic or hemorrhagic areas as in HCC. Microscopically, they are composed of normal-appearing hepatocytes arranged in plates of one or two cells thickness with areas of fatty change. While there may be some suggestion of a disordered growth pattern, there may be no evidence of nuclear atypia. The nodules are devoid of portal tracts 67.
Low-grade dysplastic nodules are defined by the absence of cellular or architectural atypia, although areas of large cell dysplasia may be present, making distinction from ordinary regenerative nodules difficult. It has been suggested that an increased number of arteries without corresponding bile ducts (so-called “unpaired arteries”) is evidence of a dysplastic nodule 69. High-grade dysplastic nodules show focal or diffuse cytologic or architectural atypia in the form of diffuse small cell dysplasia or microacinar formation. The areas of atypia appear as subnodules or nodule-in-nodule, pushing against the surrounding hepatocytes within the dysplastic nodule. These subnodules have been shown to proliferate more rapidly than the surrounding nodule and may be difficult to distinguish from well-differentiated HCC. They may also display iron resistance in an otherwise siderotic nodule, increased copper, fatty change, Mallory's hyaline, clear cell change or thickened trabeculae.
In one study, about 50% of patients with biopsy-proven dysplastic nodules developed carcinoma over a 6–50-month period 70. Cases have been described in which carcinomas were clearly embedded within adenomatous lesion, and in one report, an HBV-related carcinoma within an adenomatous lesion was shown to have an identical clonal HBV integration pattern to the surrounding hepatocytes, indicating a common origin 71. There is also evidence that these nodules are monoclonal in nature.
Nodular regenerative hyperplasia is a condition in which the entire liver is interspersed with foci of hyperplastic hepatocytes consisting of plates of more than one cell thickness in the periportal areas. In some instances these regenerative nodules progress to multiple adenomatous lesions of up to several centimeters in diameter. It is suspected that nodular regenerative hyperplasia may be a premalignant condition 72 and indeed progression of this lesion to carcinoma has been reported 73.
Liver cell dysplasia
Liver cell dysplasia refers to the presence of large hepatocytes with bizarre, hyperchromatic and occasionally multiple nuclei (large cell dysplasia). These cells occur in groups and may sometimes occupy entire cirrhotic nodules. One contention maintains that dysplastic hepatocytes represent a premalignant change, but reports from South Africa and Japan have refuted this, suggesting that liver cell dysplasia may be a regenerative or hyperplastic phenomenon 74. More recent support for the premalignant concept comes from ploidy studies, which indicate that morphologically dysplastic hepatocytes are associated with DNA aneuploidy 75,76,77, although conflicting results have also been reported 78.
Another form of liver dysplasia has been described in which the cells have small nuclei and relatively less than normal, often basophilic cytoplasm, yielding an increased nuclear to cytoplasmic ratio. This has come to be known as small cell dysplasia 79. These cells also show nuclear pleomorphism and sometimes multi-nucleation, and have been readily identified in cases from Asia associated with HCC. This association is less so among North American and European populations.
A recent study of various atypical features including large and small cell dysplasia, cytoplasmic basophilia, small microacinar structures, peripheral distribution of nuclei, nuclear irregularities, and thickened liver plates, found that when used separately, none were useful to discriminate between cirrhosis without tumor and cirrhosis associated with HCC. Acinar structures, thickened liver trabeculae, peripheral distribution of nuclei, and nuclear irregularities seemed to be the most specific indicators of association with HCC, and cirrhotic nodules showing four or more of the assessed features were often located in the vicinity of a tumor 80.
Carcinogenesis
It is accepted that neoplastic development is a stepwise process that involves at least two or more genetic events cumulating in unrestrained cell growth, tissue invasion, and metastasis. These genetic changes maybe inherited as germline mutations, which predispose to an increased risk for the development of cancer. More often they are acquired and the result of any one or a combination of chemical, physical or biological insults to the cell 81. An alternative view is that neoplastic development results from adaptive responses to environmental perturbations 82.
Colonic carcinogenesis is the best-characterized human cancer model. The so-called adenoma-carcinoma sequence in the colon formed the basis for studying the underlying molecular events and the responsible genes. While some animal models of hepatic carcinogenesis satisfy such a sequence of events, the situation in human HCC is not as well defined.
HCCs display numerous genetic abnormalities including chromosomal deletions, re-arrangements, aneuploidy, gene amplifications, and mutations, as well as epigenetic alterations including modulation of DNA methylation. Such genetic and epigenetic alterations combine to activate positive mediators of cell proliferation and inactivate negative mediators of cell proliferation including tumor suppressor genes, resulting in autonomous growth properties. Because HCCs exhibit a high degree of genetic heterogeneity it is likely that multiple molecular pathways may be involved in the production of subsets of hepatocellular tumors.
HCC has revealed allele losses from chromosomes 4, 5q, 11p, 13q, 16q, and 17p (especially the latter). Mutations of p53 have been documented in HCC-derived cell lines and in as many as 80% of liver cancers in China and southern Africa 83,84. These mutations have commonly consisted of a transversion of G to T to C at the third base of codon 249. While p53 mutations may have an important role in hepatocarcinogenesis, such mutations represent one of the most commonly recognized changes in human carcinomas and are generally a late event in carcinogenesis. Aflatoxin Bl causes transversion of G to T almost exclusively and preferentially binds to G residues in the GC-rich regions in codon 249 of the p53 gene, suggesting that this mycotoxin may have a carcinogenic role in a subset of patients with HCC.
Changes in DNA methylation have been proposed to be an essential step in carcinogenesis as they relate to the regulation of gene expression and cellular differentiation. DNA hypomethylation has been reported in chemical hepatocarcinogenesis 85 but increases in deoxycytosine methylation have been reported following ingestion of the carcinogen methapyriline 86. Prolonged feeding of diets deficient in sources of transferable methyl groups such as choline and methionine induced a high incidence of HCCinrats 87.
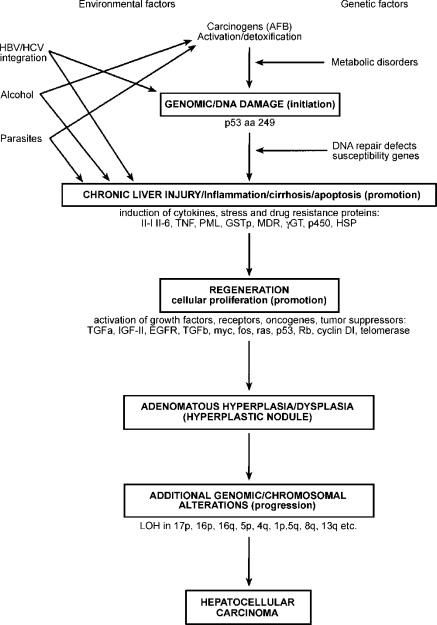
The relationship between cirrhosis and HCC is well accepted but the reason is largely unknown. It has been suggested that a deficiency in the ability to repair O(6)-methylguanine DNA underlies this increased risk, although this may be only one of several contributory factors 88. A proposed sequence of hepatocarcino-genesis is shown in Figure 289.
Figure 2. .
Proposed sequence of hepatocarcinogenesis (after Chan et al. 89).
Alcohol cannot be considered as a bona fide promoting agent for HCC and appears to act through its induction of cirrhosis as well as the modulation, in an as yet ill-defined manner, of the process of carcinogenesis with other recognized carcinogenic agents such as HBV and HCV 90. The association of HBV and HCC is strong and in addition to the supporting arguments made earlier, hepatoma cell lines have successfully produced HBV surface antigen (HBsAg) and have been demonstrated to have integrated HBV DNA. HBV DNA integration is almost invariably present in HBsAg-positive HCCs. The presence of such integration in the non-tumorous hepatocytes of these livers further indicates that integration precedes carcinogenesis; however, no oncogenes have yet been identified within the HBV genome. While the virus is frequently fragmented after it integrates into the hepatocyte genome, the HBx gene appears to be consistently retained in a functional form, leading to speculation of its role in carcinogenesis. In tissue cultures, the X protein acts as a transcriptional transactivator of viral genes and it is possible that this protein may alter host gene expression in a manner that leads to HCC formation. Transgenic mice harboring the HBx gene develop multifocal areas of altered hepatocytes, benign adenomas, and eventually HCC 91. Studies in HBx transgenic mice indicate that the HBx gene has mitogenic activity both in vitro and in vivo and suggest that the HBx gene contributes to hepatocarcinogenesis by driving cells into deregulated cell cycle control 92.
Finally, a causal association between HBV and HCC is supported by numerous studies of three hepadnaviruses, which are phylogenetically related to the human HBV. These occur in the eastern woodchuck (Marmota manax), Beechey ground squirrel (Spermo-philus beecheyi), and Peking duck (Anas domesticus), in which persistent antigenemia is associated with the development of HCC 93.
Much important information has been accumulated on the molecular and genetic events leading up to HCC, especially in the experimental model, but the genes involved and the mutations necessary for hepatocarcinogenesis still remain largely unknown 94.
References
- 1.Leong AS-Y. Leong AS-Y, Liew CT, Lau JWY, Johnson PJ. Arnold; London: 1999. Epidemiology, risk factors, etiology, premalignant lesions and carcinogenesis, Hepatocellular carcinoma. Contemporary diagnosis, investigation and management; pp. 1–29. [Google Scholar]
- 2.Rustgi VK. Epidemiology of hepatocellular carcinoma. Gastroenterol Clin North Am. 1987;16:545–51. [PubMed] [Google Scholar]
- 3.Munoz N, Bosch X. Okuda K, Ishak KG. Springer-Verlag; Berlin: 1988. Epidemiology of hepatocellular carcinoma, Neoplasms of the liver; pp. 3–19. [Google Scholar]
- 4.Simonetti RG, Camma C, Fiorello F, Politi F, D'Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962–72. doi: 10.1007/BF01297149. [DOI] [PubMed] [Google Scholar]
- 5.Okuda K, Kojiro M, Okuda H. Schiff L, Schiff ER. JB Lippincott; Philadelphia: 1993. Neoplasms of the liver, Diseases of the liver 7th ed; pp. 1236–96. [Google Scholar]
- 6.Yang BH, Xia JL, Huang LW, Tang ZY, Chen MS, Li JQ, et al. Changes of clinical aspect of primary liver cancer in China during the past 30 years—control study of 3250 cases with primary liver cancer. Zhonghua Yi Xue Za Zhi. 2003;83:1053–7. [PubMed] [Google Scholar]
- 7.Petry W, Heintges T, Hensel F, Erhardt A, Wenning M, Niederau C, et al. Hepatocellular carcinoma in Germany. Epidemiology, etiology, clinical aspects and prognosis in 100 consecutive patients of a university clinic. Z Gastroenterol. 1997;35:1059–67. [PubMed] [Google Scholar]
- 8.Oon CJ, Rauff A, Tan LKA. Treatment of primary liver cancer in Singapore. A review of 3200 cases seen between January 1, and July 31, 1987. Cancer Chemother Pharmacol 1989. 1977;23(Suppl):S13–6. doi: 10.1007/BF00647231. [DOI] [PubMed] [Google Scholar]
- 9.Wu PC. Hepatocellular carcinoma: epidemiology and pathology. Hong Kong Pract. 1983;5:790–5. [Google Scholar]
- 10.Munoz N, Linsell A. Correa P, Haenzel W. Nijhoff; The Hague: 1982. Epidemiology of primary liver cancer, Epidemiology of cancer in the digestive tract; pp. 161–95. [Google Scholar]
- 11.Lau JY-N, Lai C-L. Hepatocarcinogenesis. Trop Gastroenterol. 1990;11:9–24. [PubMed] [Google Scholar]
- 12.Tong MJ, Hwang SJ. Hepatitis B virus infection in Asian countries. Gastroenterol Clin North Am. 1994;23:523–36. [PubMed] [Google Scholar]
- 13.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:128. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Yu SZ. Primary prevention of hepatocellular carcinoma. J Gastroenterol Hepat. 1995;10:674–82. doi: 10.1111/j.1440-1746.1995.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 15.Craig JR, Peters R, Edmonson H. Tumors of the liver and intrahepatic bile ducts. Fascicle 26. Armed Forces Institute of Pathology, Washington, DC, 1989. [Google Scholar]
- 16.Saracci R, Repetto F. Time trends of primary liver cancer: indication of increased incidence in selected cancer registry populations. J Natl Cancer Inst. 1980;65:241–6. [PubMed] [Google Scholar]
- 17.Cortes-Espinosa T, Mondragon-Sanchez R, Hurtado-Andrade H, Sanchez-Cisneros R. Hepatocellular carcinoma and hepatatis in Mexico: a 25 year necropsy review. Hepatogastroenterology. 1997;44:1401–3. [PubMed] [Google Scholar]
- 18.Fan ST, Wong J. Terblanche J. Churchill Livingstone; London: 1996. Hepatocellular carcinoma in the East, Hepatobiliary malignancy; pp. 169–83. [Google Scholar]
- 19.Newberne PM, Butler WD. MIT Press; Cambridge: 1978. Rat hepatic neoplasia. [Google Scholar]
- 20.Grasso P. Williams R, Johnson PJ. Bailliere; London: 1987. Experimental liver tumours in animals, Liver tumors. Bailliere's clinical gastroenterology; pp. 183–305. [DOI] [PubMed] [Google Scholar]
- 21.Nagasue N, Kohno H, Chang Y, Hayashi T, Nakamura T. Specificity of androgen receptors of hepatocellular carcinoma and liver in humans. Hepatogastroenterology. 1990;37:474–9. [PubMed] [Google Scholar]
- 22.Eagon PK, Francavilla A, Di Leo A, Elm MS, Gennari L, Mazzaferro V, et al. Quantitation of estrogen and androgen receptors in hepatocellular carcinoma and adjacent normal human liver. Dig Dis Sci. 1991;36:1303–8. doi: 10.1007/BF01307527. [DOI] [PubMed] [Google Scholar]
- 23.Carr BI, van Thiel DH. Hormonal manipulation of human hepatocellular carcinoma. J Hepatol. 1990;11:287–9. doi: 10.1016/0168-8278(90)90209-a. [DOI] [PubMed] [Google Scholar]
- 24.D'Arville CN, Johnson PJ. Growth factors, endocrine aspects and hormonal treatment in hepatocellular carcinoma—an overview. J Steroid Biochem Mol Biol. 1990;37:1007–12. doi: 10.1016/0960-0760(90)90458-w. [DOI] [PubMed] [Google Scholar]
- 25.Tarao K, Ohkawa S, Shinmizu A, Harada M, Nakamura Y, Ito Y, et al. The male preponderance in incidence of hepatocellular carcinoma in cirrhotic patients may depend on the higher DNA synthetic activity of cirrhotic tissue in men. Cancer. 1993;72:369–74. doi: 10.1002/1097-0142(19930715)72:2<369::aid-cncr2820720210>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.See KL, See M, Glund C. Liver pathology associated with the use of anabolic-androgenic steroids. Liver. 1992;12:73–9. doi: 10.1111/j.1600-0676.1992.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 27.Moore SW, Hesseling PB, Wessels G, Schneider JW. Hepatocellular carcinoma in children. Pediatr Surg Int. 1997;12:266–70. doi: 10.1007/BF01372147. [DOI] [PubMed] [Google Scholar]
- 28.Bianchi L. Glucogen storage disease I and hepatocellular carcinoma. Eur J Pediatr. 1993;152(Suppl l):S63–70. doi: 10.1007/BF02072092. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg AG, Finegold MJ. Primary hepatic tumors in childhood. Hum Pathol. 1983;14:512–37. doi: 10.1016/s0046-8177(83)80005-7. [DOI] [PubMed] [Google Scholar]
- 30.Alberts SR, Lanier AP, McMahon BJ. Clustering of hepatocellular carcinoma in Alaska native families. Genet Epidemiol. 1991;8:127–39. doi: 10.1002/gepi.1370080206. [DOI] [PubMed] [Google Scholar]
- 31.Shen FM, Lee MK, Gong HM. Complex segregation analysis of primary hepatocellular carcinoma in Chinese families: interaction of inherited susceptibility and hepatitis B viral infection. Am J Hum Genet. 1991;49:88. [PMC free article] [PubMed] [Google Scholar]
- 32.Leuschner I, Harms D, Schmidt D. The association of hepatocellular carcinoma in childhood with hepatitis B virus infection. Cancer. 1988;62:2363–9. doi: 10.1002/1097-0142(19881201)62:11<2363::aid-cncr2820621118>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 33.Cheah PL, Looi L-M, Lim HP, Yap SF. Childhood primary hepatocellular carcinoma and hepatitis B virus infection. Cancer. 1990;65:174–6. doi: 10.1002/1097-0142(19900101)65:1<174::aid-cncr2820650133>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.Laferia G, Kaye SB, Crean GP. Hepatocellular and gastric carcinoma associated with familial polyposis coli. J Surg Oncol. 1988;38:19–21. doi: 10.1002/jso.2930380107. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein S, Scottalini AG, Loo ST. Ataxia telengectasia with hepatocellular carcinoma in a 15 year old girl and studies of her kindred. Arch Pathol Lab Med. 1985;109:1000–4. [PubMed] [Google Scholar]
- 36.McGoldrick JP, Boston VE, Glasgow JKT. Hepatocellular carcinoma associated with congenital macronodular cirrrhosis in a neonate. J Pediatr Surg. 1986;21:177–9. doi: 10.1016/s0022-3468(86)80079-3. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg AG, Mize CE, Worthan HG. The occurrence of hepatoma in the chronic form of hereditary tyrosinaemia. J Pediatr. 1976;88:434–8. doi: 10.1016/s0022-3476(76)80259-4. [DOI] [PubMed] [Google Scholar]
- 38.Dehner LP, Snover DC, Sharp HL, Sharp HL, Ascher N, Nakhleh R. Hereditary tyrosinaemia type I (chronic form): pathologic findings in the liver. Hum Pathol. 1989;20:49–58. doi: 10.1016/0046-8177(89)90179-2. [DOI] [PubMed] [Google Scholar]
- 39.Kauppinen R, Mustajoki P. Acute hepatic porphyria and hepatocellular carcinoma. Br J Cancer. 1988;57:117–20. doi: 10.1038/bjc.1988.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gangaidzo IT, Gordeuk VR. Hepatocellular carcinoma and African iron overload. Gut. 1995;37:727–30. doi: 10.1136/gut.37.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crystal RG. Alpha-1-antitrypsin deficiency, emphysema and liver disease. J Clin Invest. 1990;85:1343–5. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson IW. Membraneous obstruction of the inferior vena cava and hepatocellular carcinoma in South Africa. Gastroenterology. 1982;82:171–8. [PubMed] [Google Scholar]
- 43.Purtilo DT, Gottlieb LS. Cirrhosis and hepatoma occurring at Boston City Hospital. Cancer ;32. 1917;1973:458–62. doi: 10.1002/1097-0142(197308)32:2<458::aid-cncr2820320225>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Niederau C, Fischer R, Sonnenberg A, Stremmel W, Tram-pisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in non-cirrhotic patients with primary hematochromatosis. N Engl J Med. 1985;313:1256–62. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- 45.Chu NS, Hung TP. Geographic variations in Wilson's disease. J Neurol Sci. 1993;117:1–7. doi: 10.1016/0022-510x(93)90145-o. [DOI] [PubMed] [Google Scholar]
- 46.Shikata T. Okuda K, Peters RL. John Wiley & Sons; New York: 1987. Primary liver carcinoma and liver cirrhosis, Hepatocellular carcinoma; pp. 53–68. [Google Scholar]
- 47.Yu MC, Yuan JM, Govindarajan S, Ross RK. Epidemiology of hepatocellular carcinoma. Can J Gastroenterol. 2000;14:703–9. doi: 10.1155/2000/371801. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan DE, Reddy KR. Rising incidence of hepatocellular carcinoma: the role of hepatitis B and C; the impact on transplantation and outcomes. Clin Liver Dis. 2003;7:683–714. doi: 10.1016/s1089-3261(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 49.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 50.Melnick JL. Phillips LA. Marcel Dekker; New York: 1983. Hepatitis B virus and liver cancer, Viruses associated with human cancer; pp. 337–67. [Google Scholar]
- 51.Beasley RP, Hwang LY. Vyas GH, Dienstag JL, Hoofnagle JH. Grune and Stratton; New York: 1984. Epidemiology of hepatocellular carcinoma, Viral hepatitis and liver disease; pp. 209–24. [Google Scholar]
- 52.Blum HE, Offensperger WB, Walter E, Offensperger S, Wahl A, Zeschnigk C, et al. Hepatocellular carcinoma and hepatitis B virus infection: molecular evidence for monoclonal origin and expansion of malignantly transformed hepatocytes. J Cancer Res Clin Oncol. 1987;113:466–72. doi: 10.1007/BF00390041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govindarajan S, Craig JR, Valinluck B. Clonal origin of hepatitis B virus-associated hepatocellular carcinoma. Hum Pathol. 1988;19:403–5. doi: 10.1016/s0046-8177(88)80488-x. [DOI] [PubMed] [Google Scholar]
- 54.Goodgame B, Shaheen NJ, Galanko J, El-Serag HB. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: publication bias? Am J Gastroenterol. 2003;98:2535–42. doi: 10.1111/j.1572-0241.2003.07678.x. [DOI] [PubMed] [Google Scholar]
- 55.Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G. Hepatitis C-related carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98:2060–3. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Torres ML, Zaragoza A, Giner R, Prima J, del Olmo JA. Incidence and epidemiological factors of hepatocellular carcinoma in Valencia during the year 2000. Rev Esp Enferm Dig 2003;95:385–8381–4 [PubMed] [Google Scholar]
- 57.Fattovich G. Progression of hepatitis B and C to hepatocellular carcinoma in Western countries. Hepatogastroenterology. 1998;3:1206–13. [PubMed] [Google Scholar]
- 58.Fujioka S, Shimomura H, Iwasaki Y, Fujio K, Nakagawa H, Onishi Y, et al. Hepatitis B virus gene in liver tissue promotes hepatocellular carcinoma development in chronic hepatitis C patients. Dig Dis Sci. 2003;48:1920–4. doi: 10.1023/a:1026153800896. [DOI] [PubMed] [Google Scholar]
- 59.Matthews G, Bhagani S. The epidemiology and natural history of HIV/HBV and HIV/HCV co-infections. J HIV Ther. 2003;8:77–84. [PubMed] [Google Scholar]
- 60.Kondo F, Wada K, Nagato Y, Nakajima T, Kondo Y, Hirooka N, et al. Biopsy diagnosis of well differentiated hepatocellular carcinoma based on new morphologic criteria. Hepatology. 1989;9:751–5. doi: 10.1002/hep.1840090516. [DOI] [PubMed] [Google Scholar]
- 61.Su DL. Drinking water and liver cancer: an epidemiological approach to the etiology of this disease in China. Chinese Med J. 1979;92:748–52. [PubMed] [Google Scholar]
- 62.Willett WC, MacMahon B. Diet and cancer—an overview. N Engl J Med 1984;310:633–638, 697–703. [DOI] [PubMed] [Google Scholar]
- 63.Patterson K, Kapur SP, Chandra RS. Hepatocellular carcinoma in a non-cirrhotic infant after prolonged parenteral nutrition. J Pediatr. 1985;27:734–45. doi: 10.1016/s0022-3476(85)80360-7. [DOI] [PubMed] [Google Scholar]
- 64.Naccarato R, Farinati F. Hepatocellular carcinoma, alcohol and cirrhosis: facts and hypothesis. Dig Dis Sci. 1991;36:1137–42. doi: 10.1007/BF01297461. [DOI] [PubMed] [Google Scholar]
- 65.Kondo F, Hiroka N, Wada K, Kondo Y. Morphological clues for the diagnosis of small hepatocellular carcinomas. Virchows Arch (A) 1987;411:15–21. doi: 10.1007/BF00734509. [DOI] [PubMed] [Google Scholar]
- 66.Kondo F, Wada K, Nagato Y. Biopsy diagnosis of well differentiated hepatocellular carcinoma based on new morphologic criteria. Hepatology. 1989;9:751–5. doi: 10.1002/hep.1840090516. [DOI] [PubMed] [Google Scholar]
- 67.Sakamoto M, Hirohashi S, Tsuda H, Ino Y, Shimosato Y, Yamasaki S, et al. Increasing incidence of hepatocellular carcinoma possibly associated with non-A, non-B hepatitis in Japan, disclosed by hepatitis B virus DNA analysis of surgically resected cases. Cancer Res. 1988;48:7294–7. [PubMed] [Google Scholar]
- 68.Thiese ND. Macroregenerative (dysplastic) nodules and hepatocarcinogenesis: theoretical and clinical considerations. Semin Liver Dis. 1995;15:360–71. doi: 10.1055/s-2007-1007287. [DOI] [PubMed] [Google Scholar]
- 69.Thiese ND. Tsui WMS, Leong AS-Y, Liew CT, Ng WF. International Academy of Pathology, Hong Kong Division; Hong Kong: 1997. Precursor lesions of hepatocellular carcinoma, Pathology of liver transplantation, viral hepatitis, and tumors; pp. 87–92. [Google Scholar]
- 70.Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Okazaki N, Takayasu K, et al. Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet. 1990;336:1150–3. doi: 10.1016/0140-6736(90)92768-d. [DOI] [PubMed] [Google Scholar]
- 71.Esumi M, Aritaka T, Arii M, Suzuki K, Tanikawa K, Mizuo H, et al. Clonal origin of human hepatoma determined by integration of hepatitis B virus DNA. Cancer Res. 1986;46:5767–71. [PubMed] [Google Scholar]
- 72.Stromeyer FW, Ishak KG. Nodular transformation (nodular “regenerative” hyperplasia) of the liver. Hum Pathol. 1981;12:60–70. doi: 10.1016/s0046-8177(81)80242-0. [DOI] [PubMed] [Google Scholar]
- 73.Sogaard PE. Nodular transformation of the liver, alpha fetoprotein and hepatocellular carcinoma. Hum Pathol. 1981;12:1052–4. doi: 10.1016/s0046-8177(81)80268-7. [DOI] [PubMed] [Google Scholar]
- 74.Anthony PP. Liver cell dysplasia: what is its significance? Hepatology. 1987;7:394–6. [Google Scholar]
- 75.Gerber MA. Recent studies on the developing human hepatocellular carcinoma. Cancer Surv. 1986;5:741–63. [PubMed] [Google Scholar]
- 76.Roncalli M, Borzio M, Brando B, Colloredo G, Servida E. Abnormal DNA content in liver cell dysplasia: a flow cytometric study. Int J Cancer. 1989;44:204–7. doi: 10.1002/ijc.2910440203. [DOI] [PubMed] [Google Scholar]
- 77.Thomas RM, Bermann JJ, Yetter RA, Moore GW, Hutchins GM. Liver cell dysplasia: a DNA aneuploid lesion with distinct morphologic features. Hum Pathol. 1992;23:496–503. doi: 10.1016/0046-8177(92)90126-n. [DOI] [PubMed] [Google Scholar]
- 78.Henmi A, Uchida T, Shikata T. Karyometric analysis of liver cell dysplasia and hepatocellular carcinoma. Cancer. 1985;55:2594–9. doi: 10.1002/1097-0142(19850601)55:11<2594::aid-cncr2820551111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe S, Okita K, Harada T, Kodama T, Numa Y, Takemoto T, et al. Morphologic studies of the liver cell dysplasia. Cancer. 1983;51:2197–205. doi: 10.1002/1097-0142(19830615)51:12<2197::aid-cncr2820511208>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 80.Ojanguren I, Castella E, Ariza A, Santos J, Planas R, Bruguera M. Liver cell atypias: a comparative study in cirrhosis with and without hepatocellular carcinoma. Histopathology. 1997;30:106–12. doi: 10.1046/j.1365-2559.1997.d01-579.x. [DOI] [PubMed] [Google Scholar]
- 81.Leong AS-Y, Robbins P, Spagnolo DV. Tumor genes and their proteins in cytologic and surgical specimens. Relevance and detection systems. Diagn Cytopathol. 1995;13:411–22. doi: 10.1002/dc.2840130509. [DOI] [PubMed] [Google Scholar]
- 82.Farber E, Rubin H. Cellular adaptation in the origin and development of cancer. Cancer Res. 1991;51:2751–61. [PubMed] [Google Scholar]
- 83.Bressac B, Kew MC, Wands JR, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–31. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 84.Scorsone KA, Zhou YZ, Butel JS, Slagle BL. P53 mutations cluster at codon 249 in hepatitis B virus-positive hepatocellular carcinomas from China. Cancer Res. 1992;52:1635–8. [PubMed] [Google Scholar]
- 85.Ushijima T, Morimura K, Hosoya Y. Establishment of methylation-sensitive-representational difference analysis and isolation of hypo- and hypermethylated genomic fragments in mouse liver tumors. J Natl Acad Sci USA. 1997;94:2103–5. doi: 10.1073/pnas.94.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hernandez L, Allen PT, Poirier LA, Lijinski W. S-adeno-sylmethionine, S-adenosyl-homocysteine and DNA methylation levels in rats fed methapyriline and analogs. Carcinogenesis. 1989;10:557–62. doi: 10.1093/carcin/10.3.557. [DOI] [PubMed] [Google Scholar]
- 87.Locker J, Reddy TV, Lombardi B. DNA methylation and hepatocarcinogenesis in rats fed a choline-devoid diet. Carcinogenesis. 1986;7:1309–12. doi: 10.1093/carcin/7.8.1309. [DOI] [PubMed] [Google Scholar]
- 88.Collier JD, Bassendine MF, Burt AD. Characterization of the DNA repair enzyme for o(6)-methylguanine in cirrhosis. J Hepatol. 1996;25:158–65. doi: 10.1016/s0168-8278(96)80068-7. [DOI] [PubMed] [Google Scholar]
- 89.Chan JY-H, Lo K-W, Li H-M, Liew CT. Leong AS-Y, Liew CT, Lau JWY, Johnson PJ. Arnold; London: 1999. Molecular aspects, Hepatocellular carcinoma. Contemporary diagnosis, investigation and management; pp. 131–45. [Google Scholar]
- 90.Farber E. Alcohol and other chemicals in the development of hepatocellular carcinoma. Clin Lab Med. 1996;16:377–94. [PubMed] [Google Scholar]
- 91.Kim CM, Koike K, Saito I. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–20. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 92.Koike K. Hepatitis B virus HBx gene and hepatocarcinogenesis. Intervirology. 1995;38:134–42. doi: 10.1159/000150424. [DOI] [PubMed] [Google Scholar]
- 93.Korba BE, Wells FV, Baldwin B, Cote PJ, Tennant BC, Popper H, et al. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology. 1989;9:461–70. doi: 10.1002/hep.1840090321. [DOI] [PubMed] [Google Scholar]
- 94.Sherman M. Hepatocellular carcinoma. Gastroenterologist. 1995;3:55–6. [PubMed] [Google Scholar]