Abstract
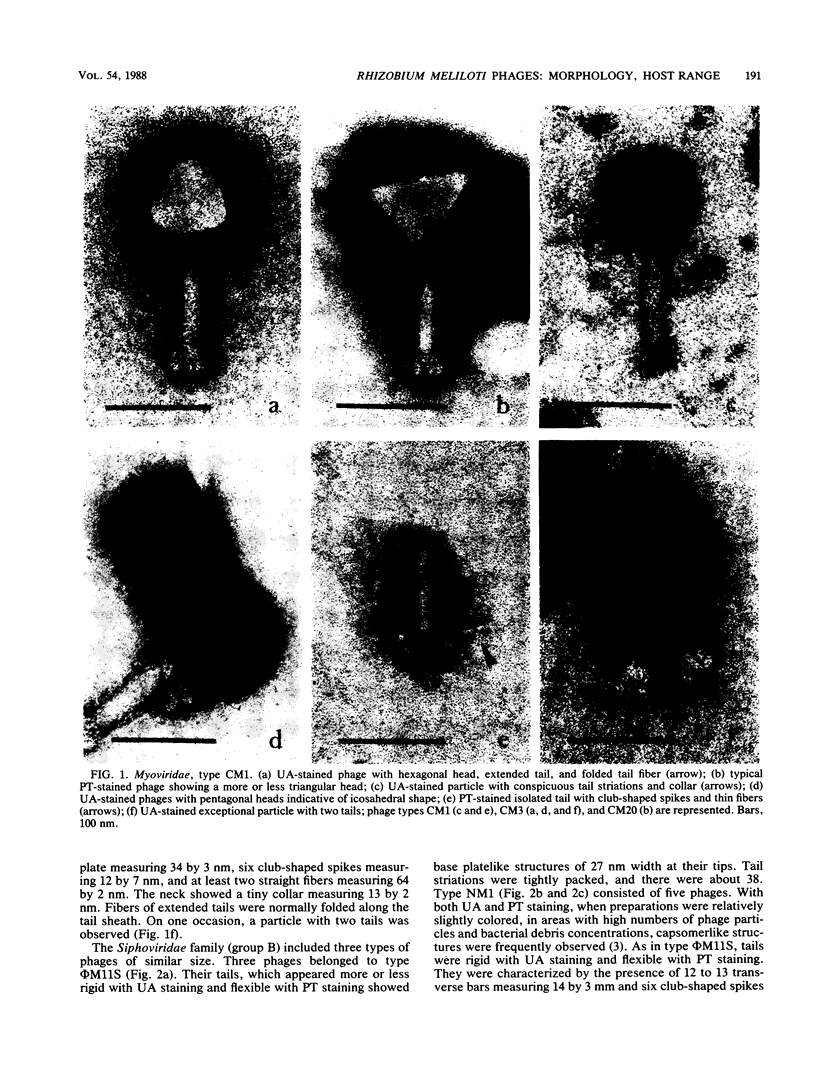
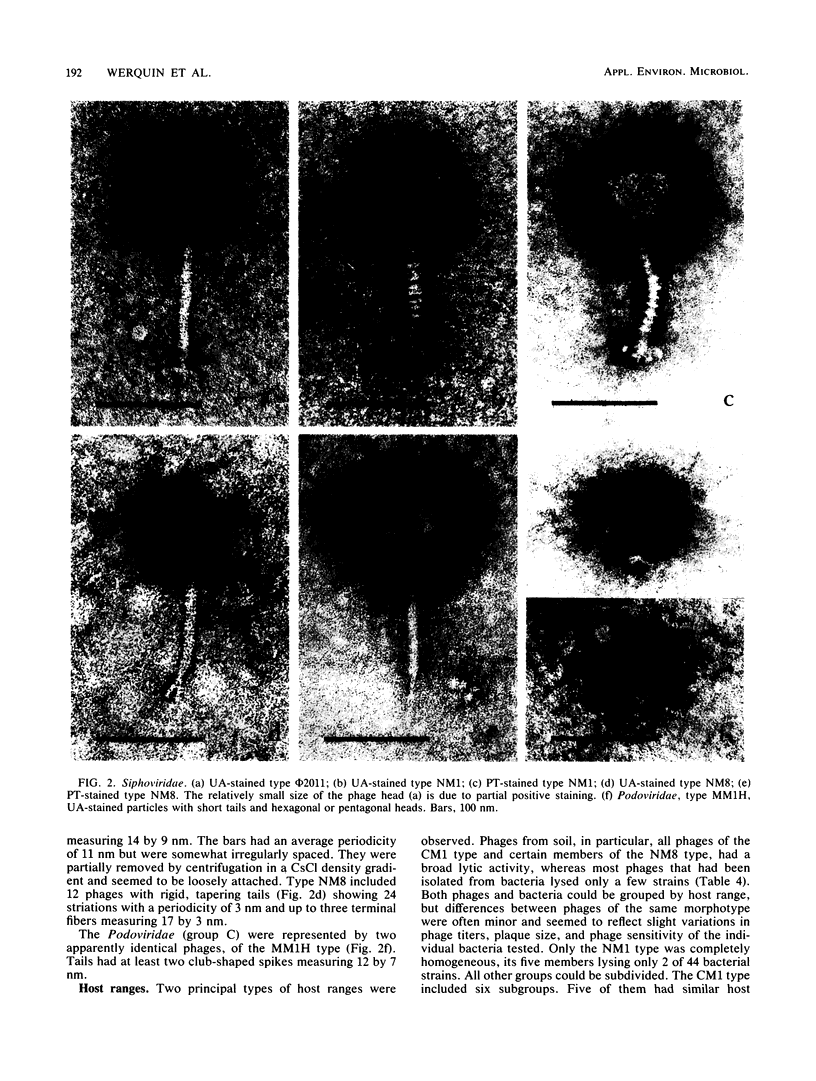
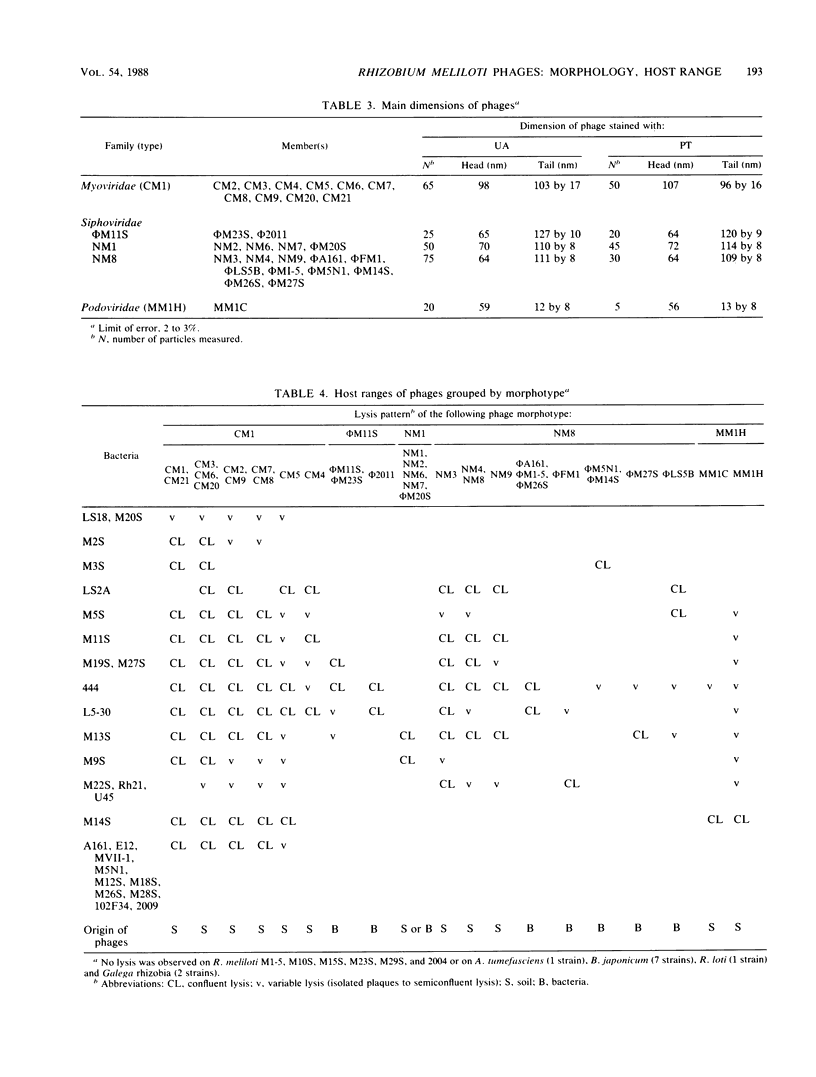
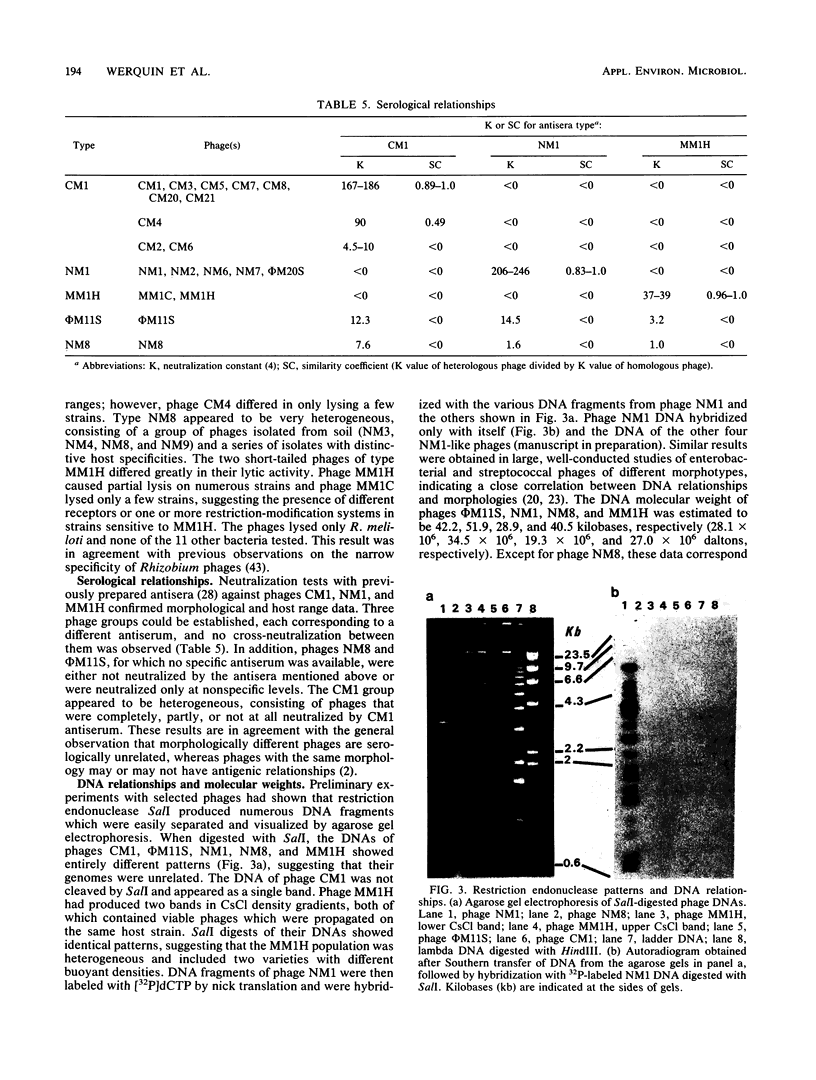
A total of 33 Rhizobium meliloti bacteriophages were studied. Of those, 21 were isolated in northern France from field soil in which Medicago sativa L. was grown. The other 12 phages were obtained by UV light and mitomycin C induction from 46 R. meliloti strains. Rhizobiophages were characterized by their morphology, host range, serological properties, restriction endonuclease patterns, DNA-DNA homologies, and DNA molecular weights. Five morphotypes were observed showing tailed phages with icosahedral heads. The categories of morphotypes included the Myoviridae (11 phages), Siphoviridae (3 morphotypes and 20 phages), and Podoviridae (2 phages). Type NM1 phage (Siphoviridae) is highly unusual because of the presence of transverse bars on the phage tail. Soil phages had broad host ranges, whereas phages isolated from bacterial cultures showed more or less narrow host ranges. Restriction endonuclease patterns and DNA-DNA hybridization experiments showed that the five phage type genomes were unrelated. Molecular weights of phage type DNAs were estimated, and they corresponded to values expected for capsid sizes, except for phage NM8. Type φM11S (Siphoviridae) did not correspond to any other described Rhizobium phages and represents a new species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann H. W., Audurier A., Berthiaume L., Jones L. A., Mayo J. A., Vidaver A. K. Guidelines for bacteriophage characterization. Adv Virus Res. 1978;23:1–24. doi: 10.1016/s0065-3527(08)60096-2. [DOI] [PubMed] [Google Scholar]
- Ackermann H. W., Jolicoeur P., Berthiaume L. Avantages et inconvénients de l'acétate d'uranyle en virologie comparée: étude de quatre bactériophages caudés. Can J Microbiol. 1974 Aug;20(8):1093–1099. [PubMed] [Google Scholar]
- Ackermann H. W. La classification des phages d'Agrobacterium et Rhizobium. Pathol Biol (Paris) 1978 Nov;26(8):507–512. [PubMed] [Google Scholar]
- Barnet Y. M. Bacteriophages of Rhizobium trifolii. I. Morphology and host range. J Gen Virol. 1972 Apr;15(1):1–15. doi: 10.1099/0022-1317-15-1-1. [DOI] [PubMed] [Google Scholar]
- Barnet Y. M. The effect of rhizobiophages on populations of Rhizobium trifolii in the root zone of clover plants. Can J Microbiol. 1980 May;26(5):572–576. doi: 10.1139/m80-101. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17(1-3):1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- Danso S. K., Keya S. O., Alexander M. Protozoa and the decline of Rhizobium populations added to soil. Can J Microbiol. 1975 Jun;21(6):884–895. doi: 10.1139/m75-131. [DOI] [PubMed] [Google Scholar]
- Dhar B., Singh B. D., Singh R. B., Srivastava J. S., Singh V. P., Singh R. M. Occurrence and distribution of rhizobiophages in Indian soils. Acta Microbiol Pol. 1979;28(4):319–324. [PubMed] [Google Scholar]
- Evans J., Barnet Y. M., Vincent J. M. Effect of a bacteriophage on colonisation and nodulation of clover roots by paired strains of Rhizobium trifolii. Can J Microbiol. 1979 Sep;25(9):974–978. doi: 10.1139/m79-149. [DOI] [PubMed] [Google Scholar]
- Evans J., Barnet Y. M., Vincent J. M. Effect of a bacteriophage on the colonisation and nodulation of clover roots by a strain of Rhizobium trifolii. Can J Microbiol. 1979 Sep;25(9):968–973. doi: 10.1139/m79-148. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Hartweig E., LeMieux K., Bergman K., Walker G. C., Signer E. R. General transduction in Rhizobium meliloti. J Bacteriol. 1984 Jul;159(1):120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiowska J., Sawicka A., Swiatek J. The occurrence of rhizobiophages in various lucerne plantations. Acta Microbiol Pol. 1976;25(2):161–163. [PubMed] [Google Scholar]
- Gregory D. W., Pirie B. J. Wetting agents for biological electron microscopy. I. General considerations and negative staining. J Microsc. 1973 Dec;99(3):251–255. doi: 10.1111/j.1365-2818.1973.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Habte M., Alexander M. Further evidence for the regulation of bacterial populations in soil by protozoa. Arch Microbiol. 1977 Jun 20;113(3):181–183. doi: 10.1007/BF00492022. [DOI] [PubMed] [Google Scholar]
- Jarvis A. W. Differentiation of lactic streptococcal phages into phage species by DNA-DNA homology. Appl Environ Microbiol. 1984 Feb;47(2):343–349. doi: 10.1128/aem.47.2.343-349.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis A. W. Serological studies of a host range mutant of a lactic streptococcal bacteriophage. Appl Environ Microbiol. 1978 Dec;36(6):785–789. doi: 10.1128/aem.36.6.785-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski M., Dénarié J. Transduction d'un géne contrôlant l'expression de la fixation de l'azote chez Rhizobium meliloti. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jul 3;275(1):141–144. [PubMed] [Google Scholar]
- Kowalski M. Transduction in Rhizobium meliloti. Acta Microbiol Pol. 1967;16(1):7–11. [PubMed] [Google Scholar]
- Krsmanovi-Simic D., Werquin M. Etude des bactériophages de Rhizobium meliloti. C R Acad Sci Hebd Seances Acad Sci D. 1973 May 9;276(19):2745–2748. [PubMed] [Google Scholar]
- Lawson K. A., Barnet Y. M., McGilchrist C. A. Environmental Factors Influencing Numbers of Rhizobium leguminosarum biovar trifolii and Its Bacteriophages in Two Field Soils. Appl Environ Microbiol. 1987 May;53(5):1125–1131. doi: 10.1128/aem.53.5.1125-1131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. An accurate measurement of the catalase crystal period and its use as an internal marker for electron microscopy. J Ultrastruct Res. 1967 Sep;20(1):91–102. doi: 10.1016/s0022-5320(67)80038-8. [DOI] [PubMed] [Google Scholar]
- Marants L. A., Moskalenko L. N., Rautenshtein Ia I. Nekotorye biologicheskie svoistva fagov Rhizobium meliloti (Liutserny. Mikrobiologiia. 1973 Nov-Dec;42(6):1088–1094. [PubMed] [Google Scholar]
- Martin M. O., Long S. R. Generalized transduction in Rhizobium meliloti. J Bacteriol. 1984 Jul;159(1):125–129. doi: 10.1128/jb.159.1.125-129.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. L., Blattner F. R. Least-squares method for restriction mapping. Gene. 1978 Oct;4(2):167–174. doi: 10.1016/0378-1119(78)90028-8. [DOI] [PubMed] [Google Scholar]
- Sik T., Horváth J., Chatterjee S. Generalized transduction in Rhizobium meliloti. Mol Gen Genet. 1980;178(3):511–516. doi: 10.1007/BF00337855. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staniewski R. Typing of Rhizobium by phages. Can J Microbiol. 1970 Oct;16(10):1003–1009. doi: 10.1139/m70-170. [DOI] [PubMed] [Google Scholar]
- Stuttard C., Dwyer M. A new temperate phage of streptomyces venezuelae: morphology, DNA molecular weight and host range of SV2. Can J Microbiol. 1981 May;27(5):496–499. doi: 10.1139/m81-073. [DOI] [PubMed] [Google Scholar]
- Vandecaveye S. C., Katznelson H. Bacteriophage as Related to the Root Nodule Bacteria of Alfalfa. J Bacteriol. 1936 May;31(5):465–477. doi: 10.1128/jb.31.5.465-477.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werquin M., Defives C., Hassani L., Andriantsimiavona-Otonia M. Large scale preparation of Rhizobium meliloti bacteriophages by fermenter culture. J Virol Methods. 1984 Feb;8(1-2):155–160. doi: 10.1016/0166-0934(84)90049-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]