Abstract
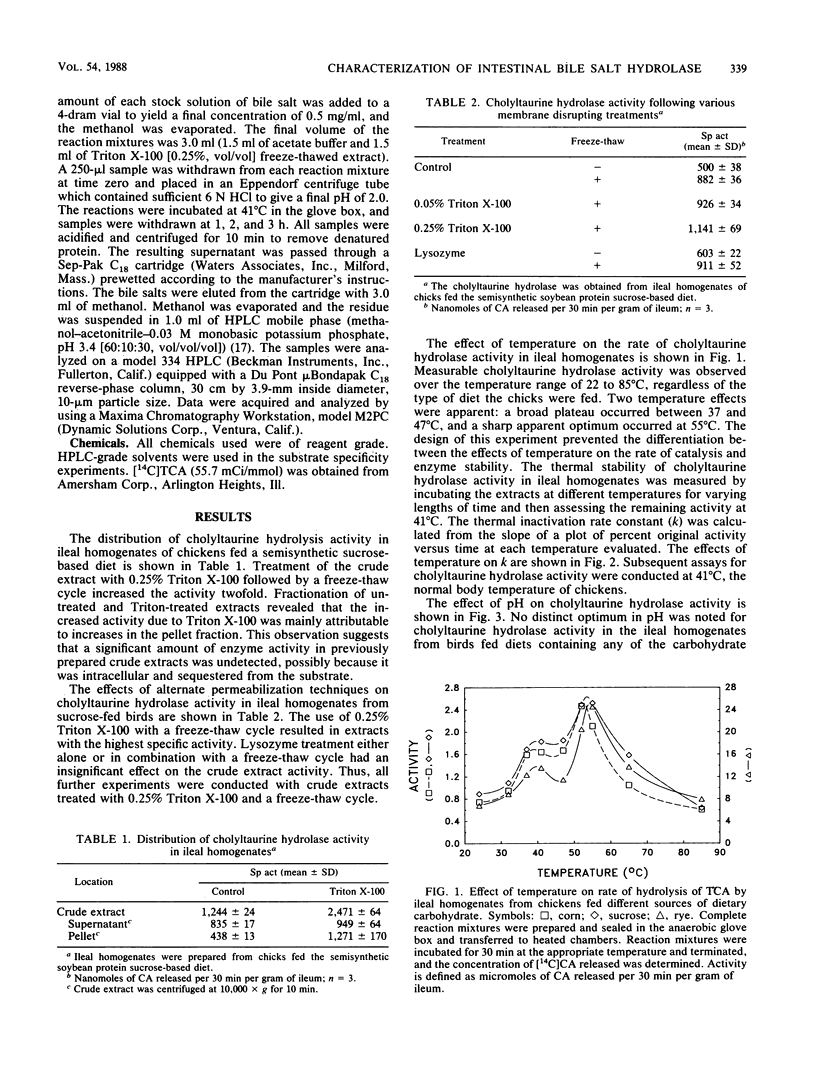
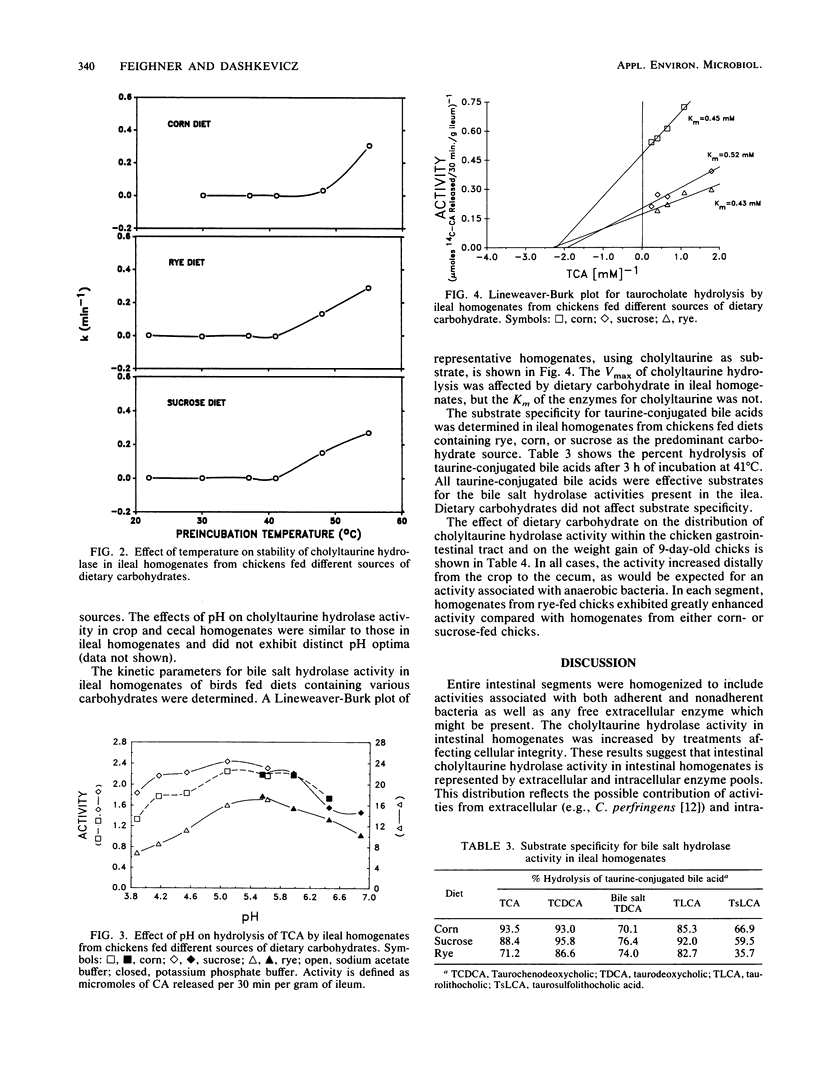
The bile salt hydrolase activity in intestinal homogenates reflects composite activities of the gastrointestinal microbial consortia. We have proposed that specific transformations of conjugated bile acids by the intestinal microflora result in the production of metabolites which depress the growth of poultry. The influence of dietary carbohydrates on the physical and kinetic properties of cholyltaurine hydrolase activity, one such bile acid-transforming enzyme in gastrointestinal homogenates of young chickens, was characterized by using a sensitive radiochemical assay. Cholyltaurine hydrolase activity in crude extracts of ileal homogenates was increased twofold by 0.25% Triton X-100 and a freeze-thaw cycle. The pH optimum for cholyltaurine hydrolase from ileal homogenates was very broad and reflected the pH range of poultry intestinal contents (i.e., 5.8 to 6.4). The carbohydrate component of the diet did not affect the apparent temperature optimum (41 degrees C) or stability profile, nor did it affect the apparent Km for taurocholic acid hydrolysis (approximately 0.43 mM). The enzymes in intestinal homogenates were active on all taurine-conjugated bile acids tested. The carbohydrate component of the diet did, however, affect the specific activity of cholyltaurine hydrolase in ileal homogenates from chickens. The levels of cholyltaurine hydrolase activity (rye greater than sucrose greater than corn) in homogenates from birds fed the different diets were directly related to the amount of growth depression (rye greater than sucrose greater than corn) associated with feeding these dietary carbohydrates. These data suggest that intestinal levels of cholyltaurine hydrolase are correlated with the amount of carbohydrate-induced growth depression in poultry.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. I. Deconjugation of bile salts. Biochim Biophys Acta. 1970 May 5;202(3):526–534. doi: 10.1016/0005-2760(70)90123-2. [DOI] [PubMed] [Google Scholar]
- Cole C. B., Fuller R. Bile acid deconjugation and attachment of chicken gut bacteria: their possible role in growth depression. Br Poult Sci. 1984 Apr;25(2):227–231. doi: 10.1080/00071668408454861. [DOI] [PubMed] [Google Scholar]
- Dickinson A. B., Gustafsson B. E., Norman A. Determination of bile acid conversion potencies of intestinal bacteria by screening in vitro and subsequent establishment in germfree rats. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):691–698. doi: 10.1111/j.1699-0463.1971.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Feighner S. D., Dashkevicz M. P. Subtherapeutic levels of antibiotics in poultry feeds and their effects on weight gain, feed efficiency, and bacterial cholyltaurine hydrolase activity. Appl Environ Microbiol. 1987 Feb;53(2):331–336. doi: 10.1128/aem.53.2.331-336.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L. Deconjugation of bile acids by intestinal lactobacilli. Appl Environ Microbiol. 1977 Jan;33(1):15–18. doi: 10.1128/aem.33.1.15-18.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Stellwag E. J. Bile acid biotransformation rates of selected gram-positive and gram-negative intestinal anaerobic bacteria. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1088–1094. doi: 10.1016/0006-291x(76)90484-8. [DOI] [PubMed] [Google Scholar]
- Kobashi K., Nishizawa I., Yamada T., Hase J. A new hydrolase specific for taurine-conjugates of bile acids. J Biochem. 1978 Aug;84(2):495–497. doi: 10.1093/oxfordjournals.jbchem.a132152. [DOI] [PubMed] [Google Scholar]
- Masuda N. Deconjugation of bile salts by Bacteroids and Clostridium. Microbiol Immunol. 1981;25(1):1–11. doi: 10.1111/j.1348-0421.1981.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Midtvedt T., Norman A. Anaerobic, bile acid transforming microorganisms in rat intestinal content. Acta Pathol Microbiol Scand. 1968;72(2):337–344. doi: 10.1111/j.1699-0463.1968.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Midtvedt T., Norman A. Bile acid transformations by microbial strains belonging to genera found in intestinal contents. Acta Pathol Microbiol Scand. 1967;71(4):629–638. doi: 10.1111/j.1699-0463.1967.tb05183.x. [DOI] [PubMed] [Google Scholar]
- Miozzari G. F., Niederberger P., Hütter R. Permeabilization of microorganisms by Triton X-100. Anal Biochem. 1978 Oct 1;90(1):220–233. doi: 10.1016/0003-2697(78)90026-x. [DOI] [PubMed] [Google Scholar]
- NORMAN A., WIDSTROEM O. A. HYDROLYSIS OF CONJUGATED BILE ACIDS BY EXTRACELLULAR ENZYMES PRESENT IN RAT INTESTINAL CONTENTS. Proc Soc Exp Biol Med. 1964 Nov;117:442–444. doi: 10.3181/00379727-117-29603. [DOI] [PubMed] [Google Scholar]
- Nair P. P., Gordon M., Reback J. The enzymatic cleavage of the carbon-nitrogen bond in 3-alpha, 7-alpha, 12-alpha-trihydroxy-5-beta-cholan-24-oylglycine. J Biol Chem. 1967 Jan 10;242(1):7–11. [PubMed] [Google Scholar]
- Nakayama F., Nakagaki M. Quantitative determination of bile acids in bile with reversed-phase high-performance liquid chromatography. J Chromatogr. 1980 Sep 12;183(3):287–293. doi: 10.1016/s0378-4347(00)81708-9. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Mallett A. K., Wise A. The effect of diet on the mammalian gut flora and its metabolic activities. Crit Rev Toxicol. 1985;16(1):31–103. doi: 10.3109/10408448509041324. [DOI] [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirehead P. A., Maglio M., Goodman J. R. Bacteria isolated from the duodenum, ileum, and cecum of young chicks. Appl Environ Microbiol. 1978 Apr;35(4):782–790. doi: 10.1128/aem.35.4.782-790.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. The development of the flora of the alimentary tract in young animals. J Pathol Bacteriol. 1965 Oct;90(2):495–513. [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. Purification and characterization of bile salt hydrolase from Bacteroides fragilis subsp. fragilis. Biochim Biophys Acta. 1976 Nov 8;452(1):165–176. doi: 10.1016/0005-2744(76)90068-1. [DOI] [PubMed] [Google Scholar]
- Stutz M. W., Johnson S. L., Judith F. R. Effects of diet and bacitracin on growth, feed efficiency, and populations of Clostridium perfringens in the intestine of broiler chicks. Poult Sci. 1983 Aug;62(8):1619–1625. doi: 10.3382/ps.0621619. [DOI] [PubMed] [Google Scholar]
- Stutz M. W., Johnson S. L., Judith F. R., Miller B. M. In vitro and in vivo evaluations of the antibiotic efrotomycin. Poult Sci. 1983 Aug;62(8):1612–1618. doi: 10.3382/ps.0621612. [DOI] [PubMed] [Google Scholar]
- Stutz M. W., Lawton G. C. Effects of diet and antimicrobials on growth, feed efficiency, intestinal Clostridium perfringens, and ileal weight of broiler chicks. Poult Sci. 1984 Oct;63(10):2036–2042. doi: 10.3382/ps.0632036. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Thomas O. P. Influence of diets containing rye or pectin on the intestinal flora of chicks. Poult Sci. 1978 Jul;57(4):971–975. doi: 10.3382/ps.0570971. [DOI] [PubMed] [Google Scholar]
- Webling D. D. The site of absorption of taurocholate in chicks, using polyethylene glycol as a reference substance. Aust J Exp Biol Med Sci. 1966 Feb;44(1):101–104. doi: 10.1038/icb.1966.11. [DOI] [PubMed] [Google Scholar]


