Abstract
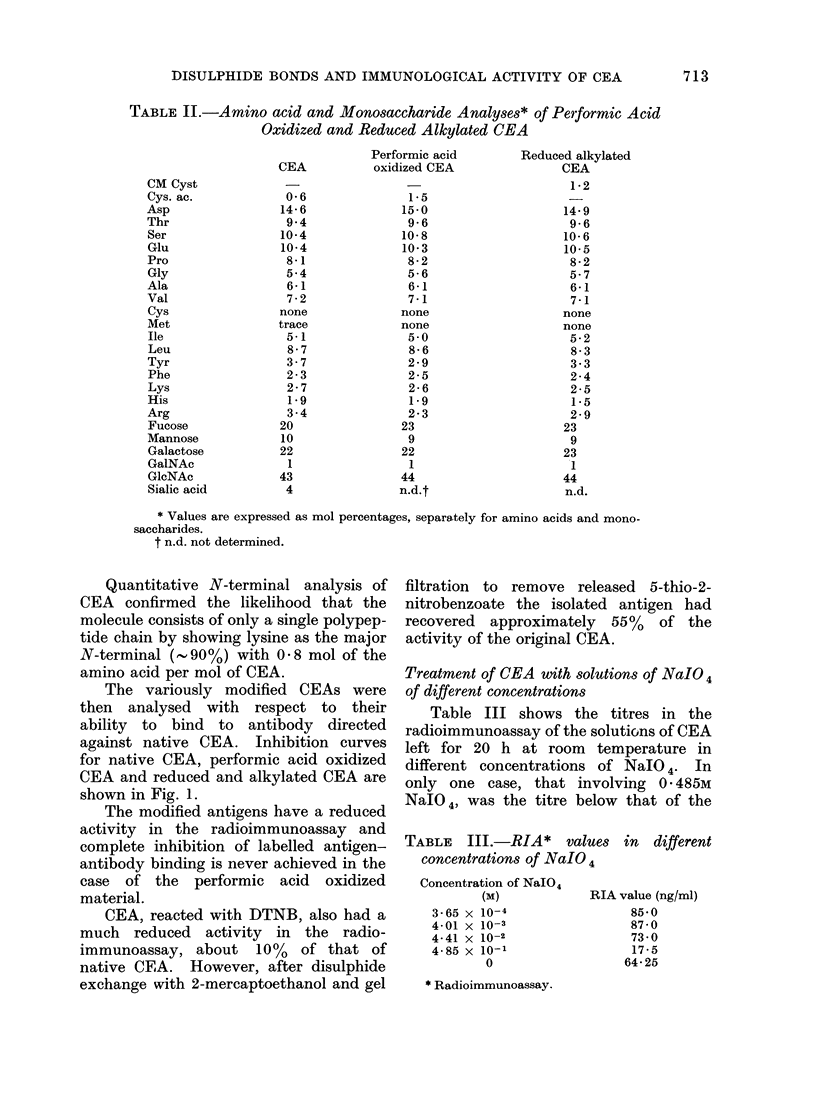
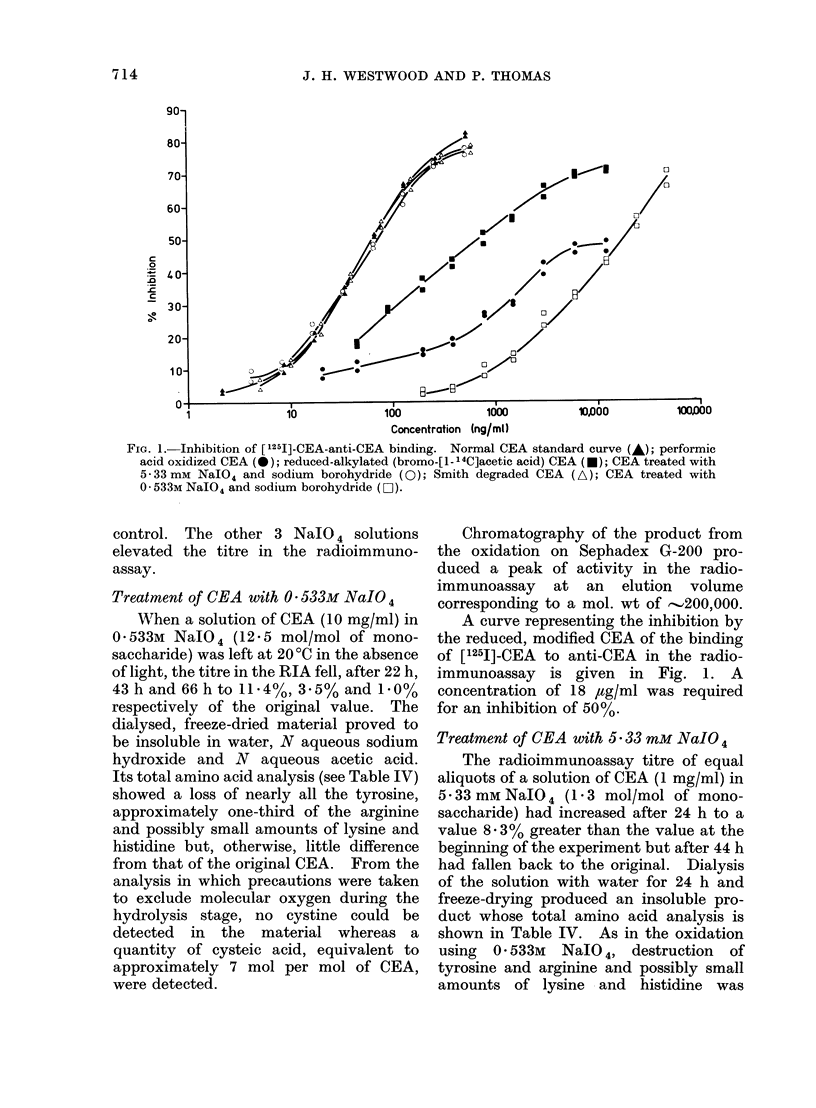
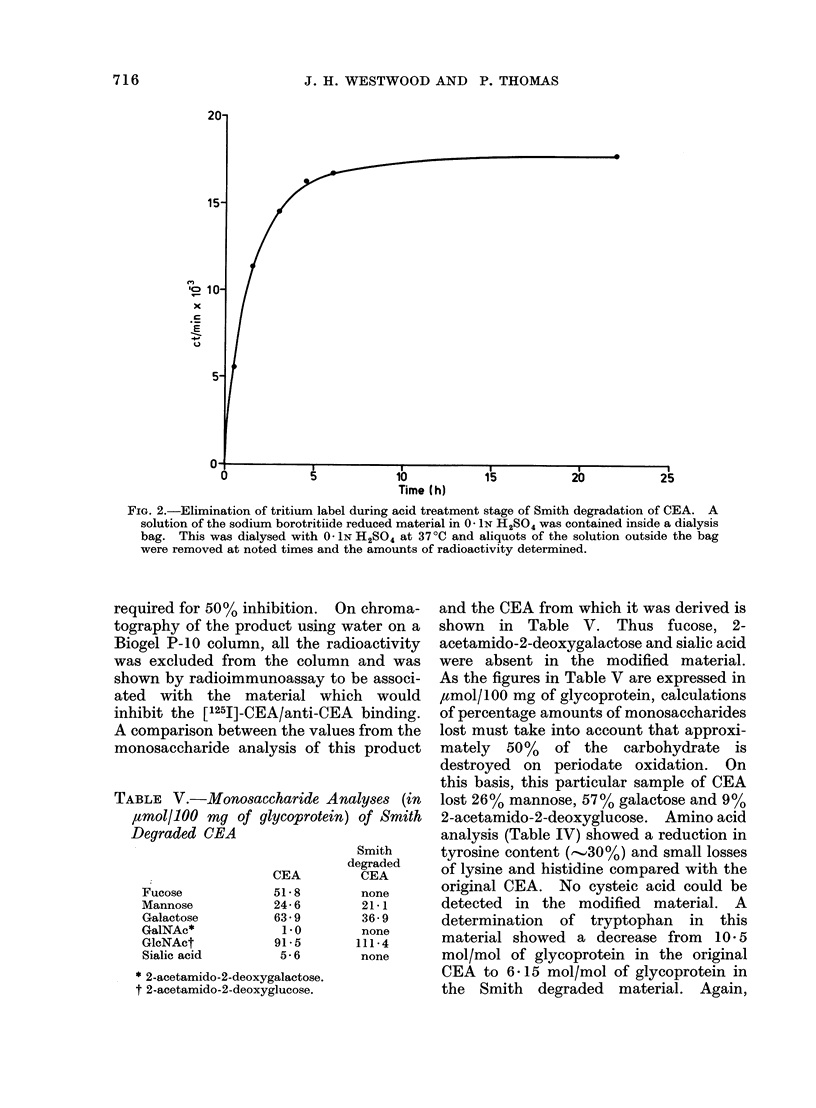
Carcinoembryonic antigen (CEA) has been shown to contain no free cysteine thiol groups but 6 cystine disulphide bonds. 5'5-Dithiobis-(2-nitrobenzoic acid) (DTNB) will react with CEA only after reduction of the disulphide bonds with dithioerythritol. Reduction-alkylation of CEA using dithioerythritol and bromo-[1-14C] acetic acid confirmed the presence of 6 disulphide bonds, as did oxidation of the glycoprotein with performic acid. The products from the DTNB and reduction-alkylation treatments of CEA had less capacity to inhibit the binding of [125I]-CEA to anti-CEA in a radioimmunoassay than the original CEA but could, in sufficient quantities, totally inhibit the binding. Removal, using mercaptoethanol, of the thiol blocking groups from the DTNB-treated CEA resulted in a 55% recovery of antigenic activity. The product from the performic acid oxidation could only inhibit approximately 50% of the binding. Treatment of CEA with 0.533M sodium periodate (NaIO4) greatly reduced its antigenic activity, presumably a result of the oxidative cleavage of the disulphide bonds. No loss in activity, however, was observed when 5.33mM NaIO4 was used, and one Smith degradation (i.e. treatment in sequence with periodate, borohydride and mild acid) of CEA removed approximately 50% of the carbohydrate, including all of the fucose, sialic acid and 2-acetamido-2-deoxygalactose but did not change the antigenic activity.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Bais R., Greenwell P., Wallace J. C., Keech D. B. Influence of sodium dodecyl sulphate on the sedimentation velocity of proteins. FEBS Lett. 1974 Apr 15;41(1):53–57. doi: 10.1016/0014-5793(74)80952-x. [DOI] [PubMed] [Google Scholar]
- Banjo C., Gold P., Freedman S. O., Krupey J. Immunologically active heterosaccharides of carcinoembryonic antigen of human digestive system. Nat New Biol. 1972 Aug 9;238(84):183–185. doi: 10.1038/newbio238183a0. [DOI] [PubMed] [Google Scholar]
- Banjo C., Gold P., Gehrke C. W., Freedman S. O., Krupey J. Preparation and isolation of immunologically active glycopeptides from carcinoembryonic antigen (CEA). Int J Cancer. 1974 Feb 15;13(2):151–163. doi: 10.1002/ijc.2910130202. [DOI] [PubMed] [Google Scholar]
- CLAMP J. R., HOUGH L. THE PERIODATE OXIDATION OF AMINO ACIDS WITH REFERENCE TO STUDIES ON GLYCOPROTEINS. Biochem J. 1965 Jan;94:17–24. doi: 10.1042/bj0940017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Henkart P. A., Todd C. W., Terry W. D. Heterogeneity of the carcinoembryonic antigen. Immunochemistry. 1973 Sep;10(9):591–599. doi: 10.1016/0019-2791(73)90160-2. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Todd C. W. Structural studies on carcinoembryonic antigen. Periodate oxidation. Biochemistry. 1975 Feb 25;14(4):805–810. doi: 10.1021/bi00675a025. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Engvall E., Johansson B. G., Svensson S., Sundblad G., Goldstein I. J. Nature of the tumor-associated determinant(s) of carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1528–1532. doi: 10.1073/pnas.72.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEANLOZ R. W., FORCHIELLI E. Studies on hyaluronic acid and related substances. II. Periodate oxidation of glucosamine and derivatives. J Biol Chem. 1951 Jan;188(1):361–369. [PubMed] [Google Scholar]
- Krupey J., Gold P., Freedman S. O. Physicochemical studies of the carcinoembryonic antigens of the human digestive system. J Exp Med. 1968 Sep 1;128(3):387–398. doi: 10.1084/jem.128.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence D. J., Stevens U., Bettelheim R., Darcy D., Leese C., Turberville C., Alexander P., Johns E. W., Neville A. M. Role of plasma carcinoembryonic antigen in diagnosis of gastrointestinal, mammary, and bronchial carcinoma. Br Med J. 1972 Sep 9;3(5827):605–609. doi: 10.1136/bmj.3.5827.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu T., Staebler D., Chavanel G., Burtin P. Study of the antigenic cross reactivity between carcinoembryonic antigen and "nonspecific cross reacting antigens" (NCA and NCA 2). Br J Cancer. 1975 May;31(5):524–527. doi: 10.1038/bjc.1975.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARK G. R., SMYTH D. G. The use of cyanate for the determination of NH2-terminal residues in proteins. J Biol Chem. 1963 Jan;238:214–226. [PubMed] [Google Scholar]
- Schmid K., Bürgi W., Collins J. H., Nanno S. The disulfide bonds of alpha1-acid glycoprotein. Biochemistry. 1974 Jun 18;13(13):2694–2697. doi: 10.1021/bi00710a006. [DOI] [PubMed] [Google Scholar]
- Terry W. D., Henkart P. A., Coligan J. E., Todd C. W. Carcinoembryonic antigen: characterization and clinical applications. Transplant Rev. 1974;20(0):100–129. doi: 10.1111/j.1600-065x.1974.tb00143.x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Watanabe K., Hakomori S. A glycosphingolipid sharing reactivity with both wheat germ lectin and "carcinoembryonic antisera (Gold)": partial identity of these reactive sites. FEBS Lett. 1973 Dec 1;37(2):317–320. doi: 10.1016/0014-5793(73)80486-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Westwood J. H., Bessell E. M., Bukhari M. A., Thomas P., Walker J. M. Studies on the structure of the carcinoembryonic antigen. I. some deductions on the basis of chemical degradations. Immunochemistry. 1974 Dec;11(12):811–818. doi: 10.1016/0019-2791(74)90302-4. [DOI] [PubMed] [Google Scholar]


