Abstract
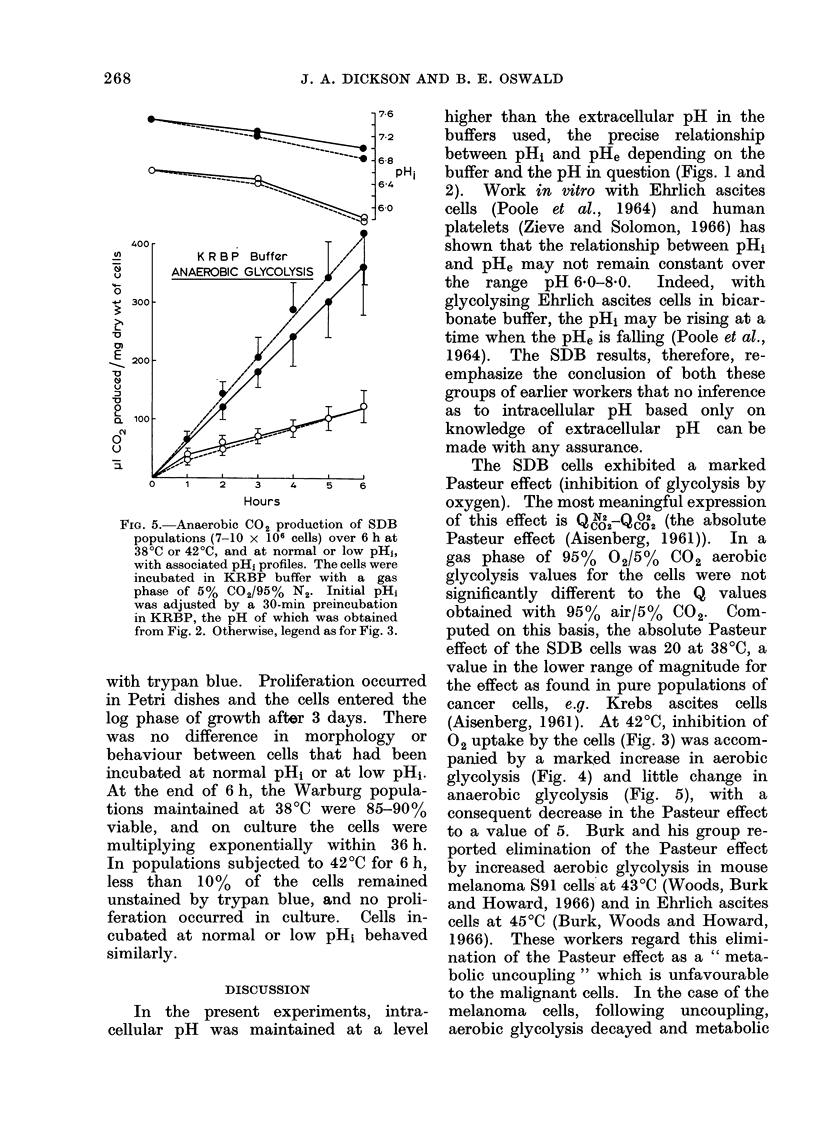
The postulate that low intracellular pH acts as a preconditioner for the destructuve effects of hyperthermia (42 degrees C) was examined, using a heat-sensitive line of malignant cells derived from rat mammary gland (SDB). Intracellular pH (pHi) was measured indirectly, from the distribution of the weak, non-metabolizable organic acid 5,5-dimethyl-2,4-oxazolidinedione (DMO) between intra- and extra-cellular water. Respiration, aerobic and anaerobic and anaerobic glycolysis of the cells were studied at normal pHi (pH 7-0-7-4) or at low pHi (pH 6-2-6-6) and at 38 degrees C or 42 degrees C over 6 h in Warburg manometers; the ability of the cells to replicate in culture was examined after 3 h or 6 h incubation in the flasks. The relationship between pHi and extracellular pH (pHe) depended upon the buffer system used and the exact pH in question; no assumption regarding pHi based only on pHe measurement could be made. At 38 degrees C and low pHi, the Pasteur effect became negative due to a relatively greater inhibition of anaerobic than aerobic glycolysis. Respiration was unaffected and cell replicative ability unimpaired. At 42 degrees C and normal pHi, respiration was totally inhibited after 4 h and the Pasteur effect was decreased, in this case due to a compensatory increase in aerobic glycolysis without alteration in anaerobic CO2 production. Low pHi in the presence of hyperthermia enabled cell respiration to continue at a reduced level with no further change in glycolysis. There was delayed cell replication after 3 h at 42 degrees C and inability to multiply following 6 h hyperthermia: low pHi did not influence these results. It is concluded that with these cancer cells, pHi values maintained in the region of 1-0 pH unit below normal for 6 h had no deleterious effect on the cells. No sensitizing effect of the low pHi for the destructive effect of hyperthermia on the cells was observed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickis I. J., Henderson I. W. Biochemical studies of human tumors. I. Estimation of tumor malignancy from metabolic measurements in vitro. Cancer. 1966 Jan;19(1):89–102. doi: 10.1002/1097-0142(196601)19:1<89::aid-cncr2820190110>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Butler T. C., Waddell W. J., Poole D. T. Intracellular pH based on the distribution of weak electrolytes. Fed Proc. 1967 Sep;26(5):1327–1332. [PubMed] [Google Scholar]
- Cavaliere R., Ciocatto E. C., Giovanella B. C., Heidelberger C., Johnson R. O., Margottini M., Mondovi B., Moricca G., Rossi-Fanelli A. Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer. 1967 Sep;20(9):1351–1381. doi: 10.1002/1097-0142(196709)20:9<1351::aid-cncr2820200902>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dickson J. A., Ellis H. A. The influence of tumor volume and the degree of heating on the response of the solid Yoshida sarcoma to hyperthermia (40-42 degrees). Cancer Res. 1976 Mar;36(3):1188–1195. [PubMed] [Google Scholar]
- Dickson J. A. Letter: Hyperthermia in the treatment of cancer. Cancer Chemother Rep. 1974 May-Jun;58(3):294–296. [PubMed] [Google Scholar]
- Dickson J. A., Muckle D. S. Total-body hyperthermia versus primary tumor hyperthermia in the treatment of the rabbit VX-2 carcinoma. Cancer Res. 1972 Sep;32(9):1916–1923. [PubMed] [Google Scholar]
- Dickson J. A., Shah D. M. The effects of hyperthermia (42 degrees C) on the biochemistry and growth of a malignant cell line. Eur J Cancer. 1972 Oct;8(5):561–571. doi: 10.1016/0014-2964(72)90110-7. [DOI] [PubMed] [Google Scholar]
- Dickson J. A., Suzangar M. A predictive in vitro assay for the sensitivity of human solid tumours to hyperthermia (42 degrees C) and its value in patient management. Clin Oncol. 1976 Jun;2(2):141–155. [PubMed] [Google Scholar]
- Dickson J. A., Suzangar M. In vitro-in vivo studies on the susceptibility of the solid Yoshida sarcoma to drugs and hyperthermia (42 degrees). Cancer Res. 1974 Jun;34(6):1263–1274. [PubMed] [Google Scholar]
- EDEN M., HAINES B., KAHLER H. The pH of rat tumors measured in vivo. J Natl Cancer Inst. 1955 Oct;16(2):541–556. [PubMed] [Google Scholar]
- IYPE P. T., BHARGAVA P. M. THE RESPIRATION OF ISOLATED RAT-HEPATIC CELLS IN SUSPENSION. Biochem J. 1965 Jan;94:284–288. doi: 10.1042/bj0940284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondovì B., Strom R., Rotilio G., Finazzi Agrò A., Cavaliere R., Rossi Fanelli A. The biochemical mechanism of selective heat sensitivity of cancer cells. I. Studies on cellular respiration. Eur J Cancer. 1969 May;5(2):129–136. doi: 10.1016/0014-2964(69)90059-0. [DOI] [PubMed] [Google Scholar]
- Overgaard K., Overgaard J. Investigations on the possibility of a thermic tumour therapy. I. Short-wave treatment of a transplanted isologous mouse mammary carcinoma. Eur J Cancer. 1972 Feb;8(1):65–78. doi: 10.1016/0014-2964(72)90085-0. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Poole D. T. Intracellular pH of the Ehrlich ascites tumour cell as it is affected by sugars and sugar derivatives. J Biol Chem. 1967 Aug 25;242(16):3731–3736. [PubMed] [Google Scholar]
- RINALDINI L. M. An improved method for the isolation and quantitative cultivation of embryonic cells. Exp Cell Res. 1959 Mar;16(3):477–505. doi: 10.1016/0014-4827(59)90119-3. [DOI] [PubMed] [Google Scholar]
- Robson J. S., Bone J. M., Lambie A. T. Intracellular pH. Adv Clin Chem. 1968;11:213–275. doi: 10.1016/s0065-2423(08)60060-8. [DOI] [PubMed] [Google Scholar]
- SCHLOERB P. R., BLACKBURN G. L., GRANTHAM J. J., MALLARD D. S., CAGE G. K. INTRACELLULAR PH AND BUFFERING CAPACITY OF THE WALKER-256 CARCINOMA. Surgery. 1965 Jul;58:5–11. [PubMed] [Google Scholar]
- Snow C., Allen A. The release of radioactive nucleic acids and mucoproteins by trypsin and ethylenediaminetetra-acetate treatment of baby-hamster cells in tissue culture. Biochem J. 1970 Oct;119(4):707–714. doi: 10.1042/bj1190707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Zieve P. D., Solomon H. M. The intracellular pH of the human platelet. J Clin Invest. 1966 Aug;45(8):1251–1254. doi: 10.1172/JCI105431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ardenne M. Selective multiphase cancer therapy: conceptual aspects and experimental basis. Adv Pharmacol Chemother. 1972;10:339–380. doi: 10.1016/s1054-3589(08)60527-x. [DOI] [PubMed] [Google Scholar]


