Abstract
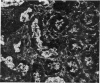
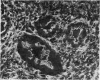
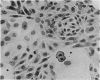
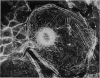
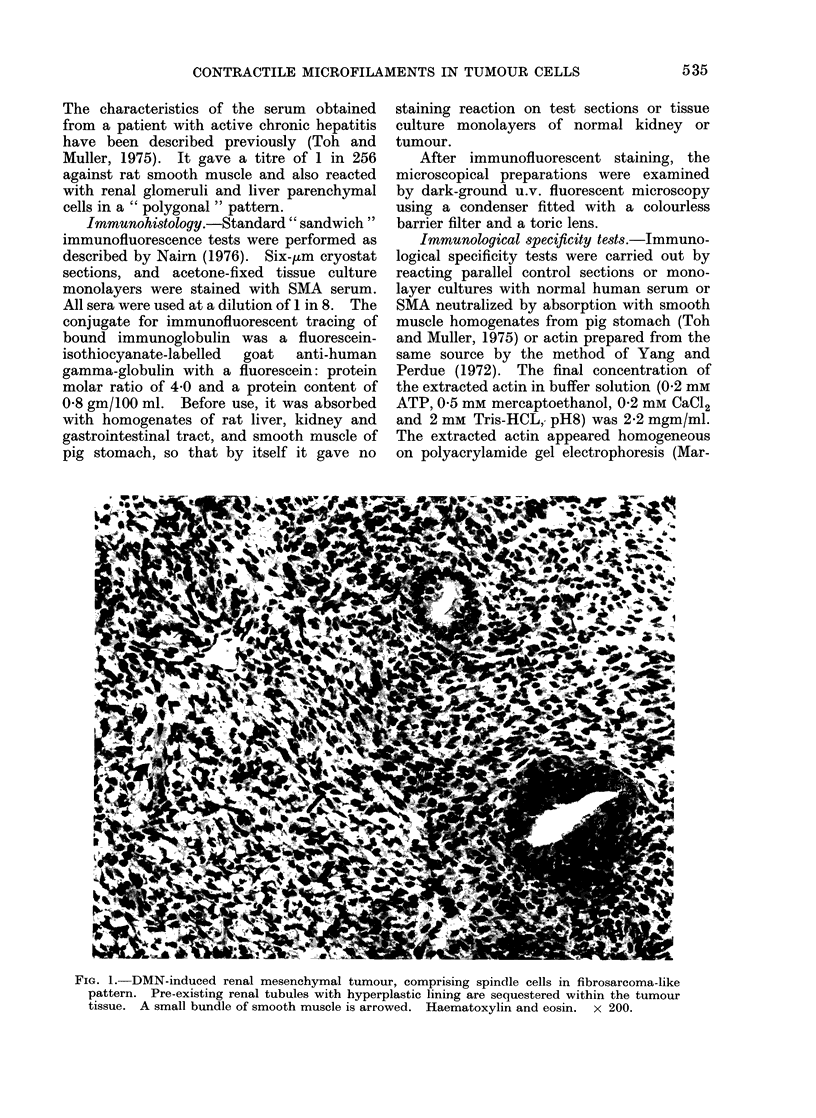
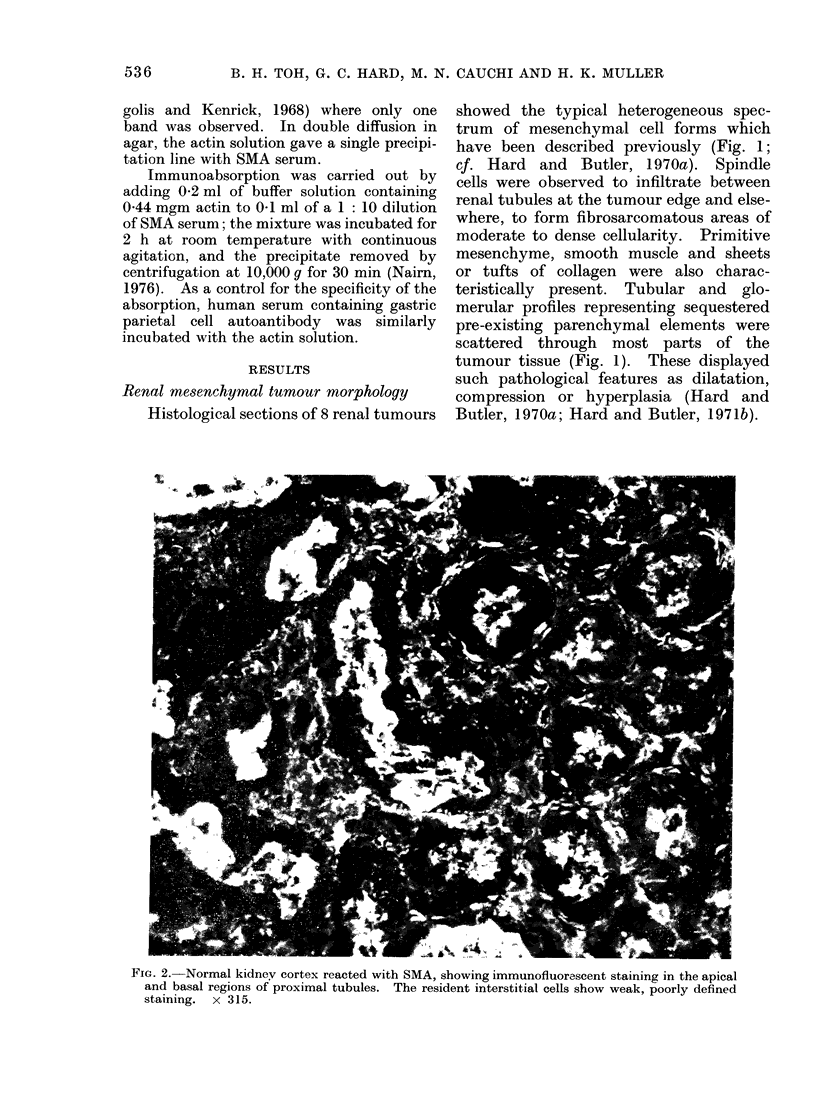
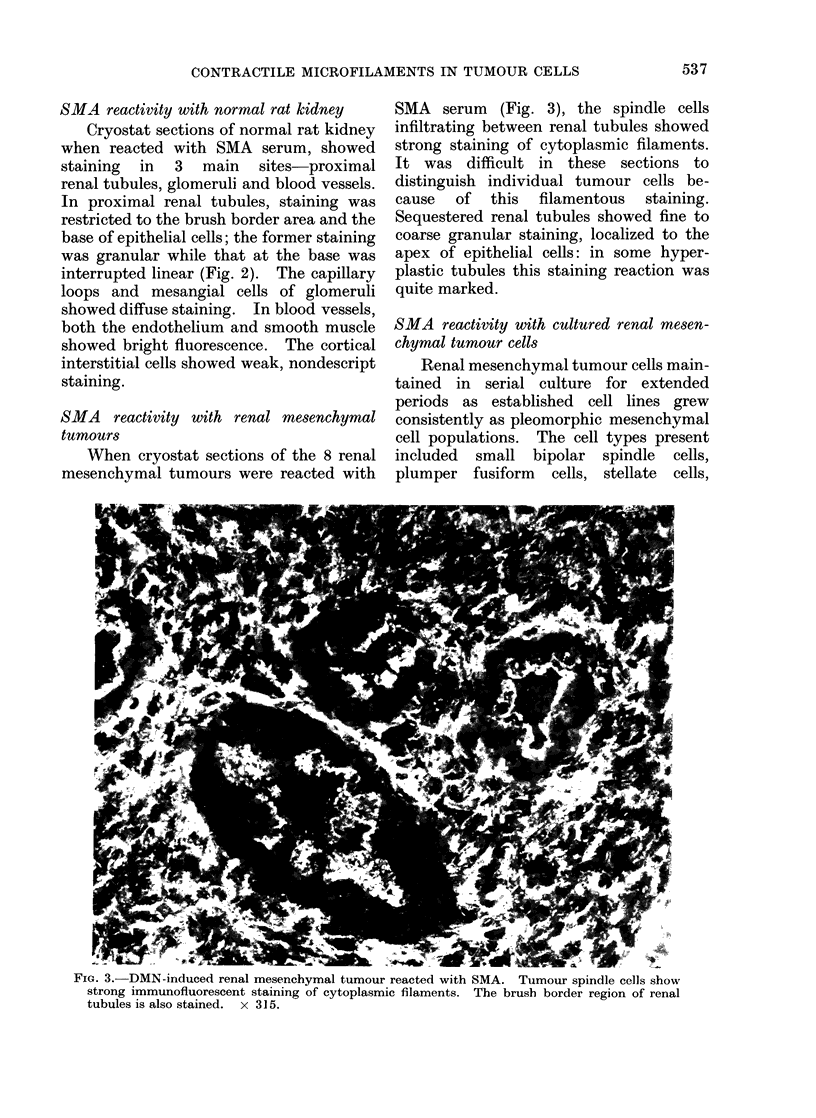
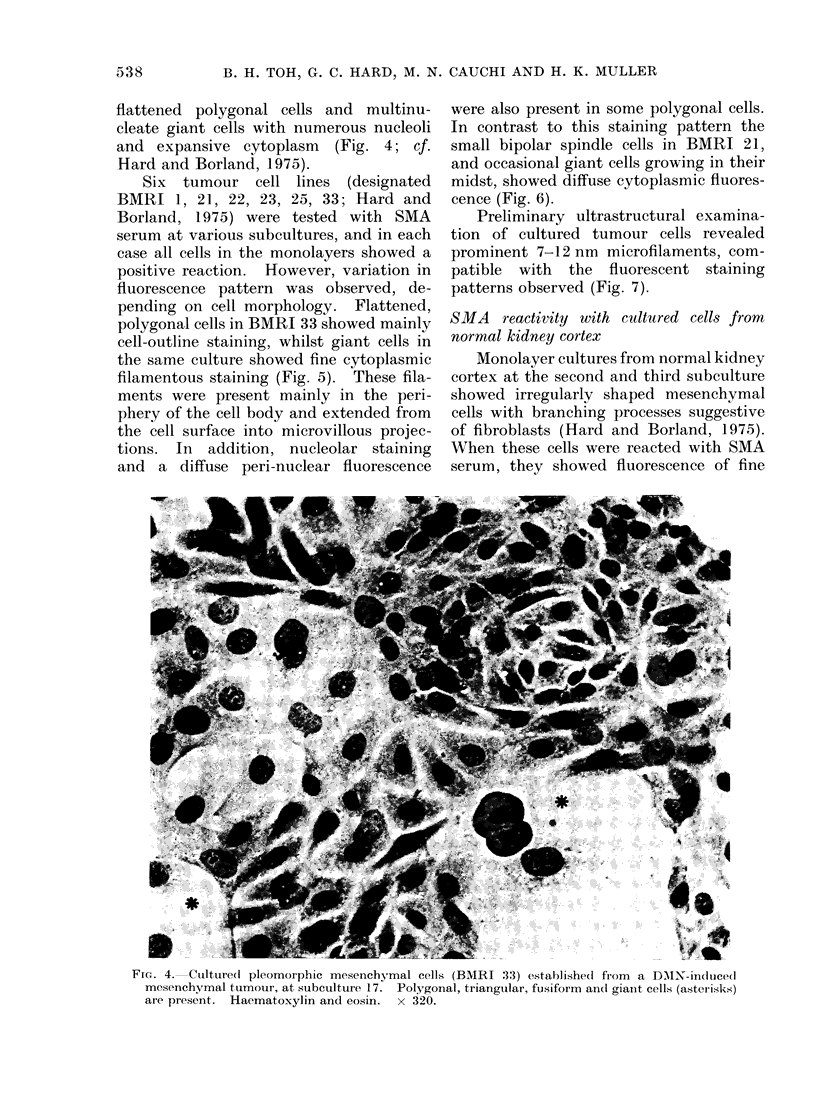
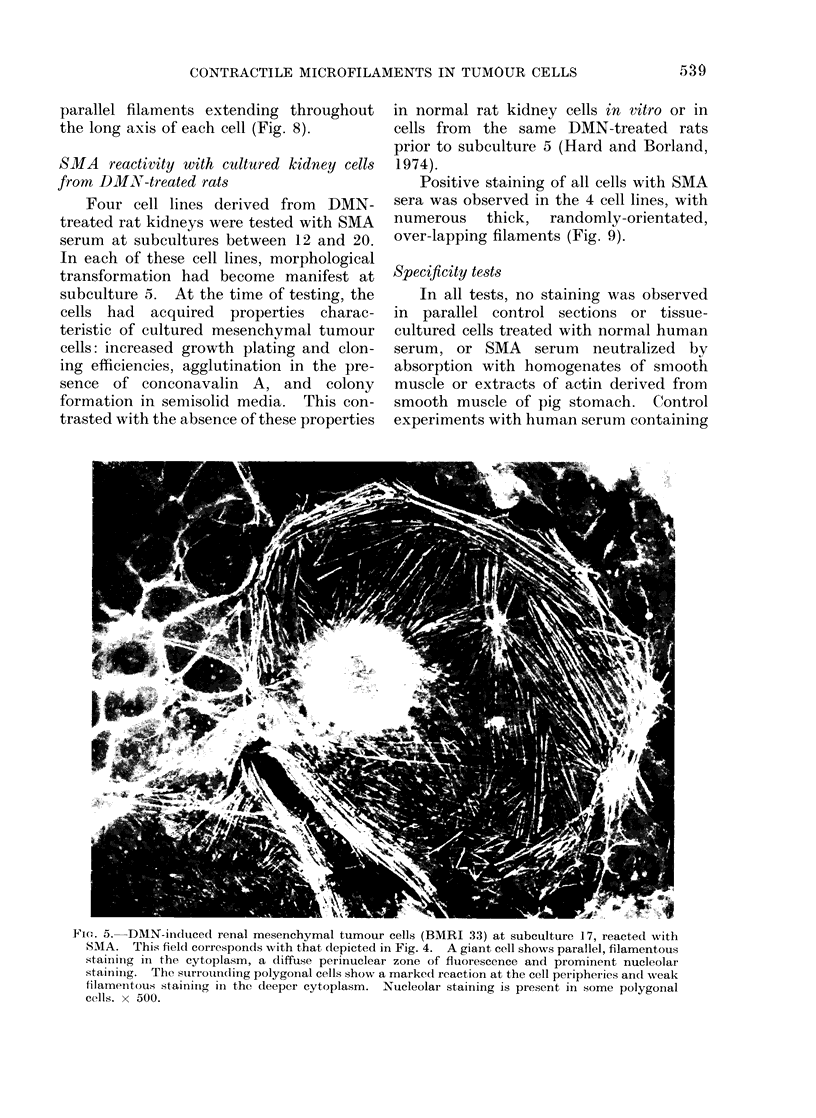
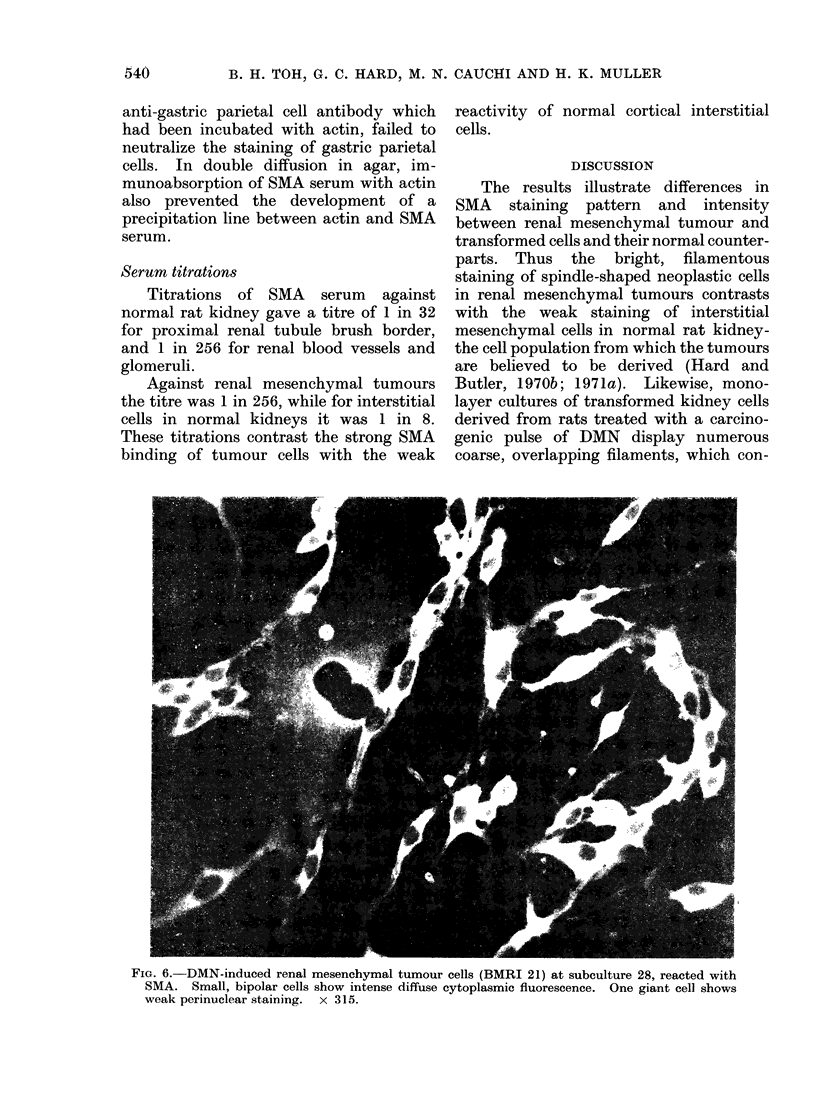
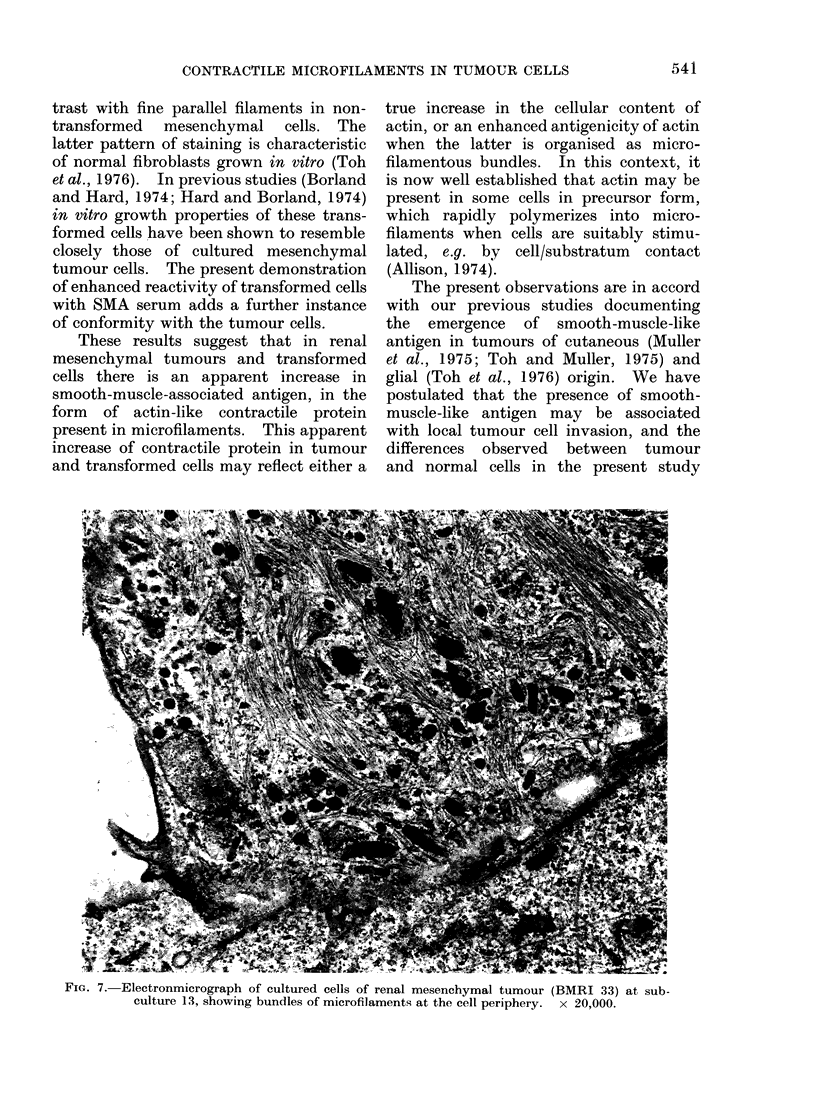
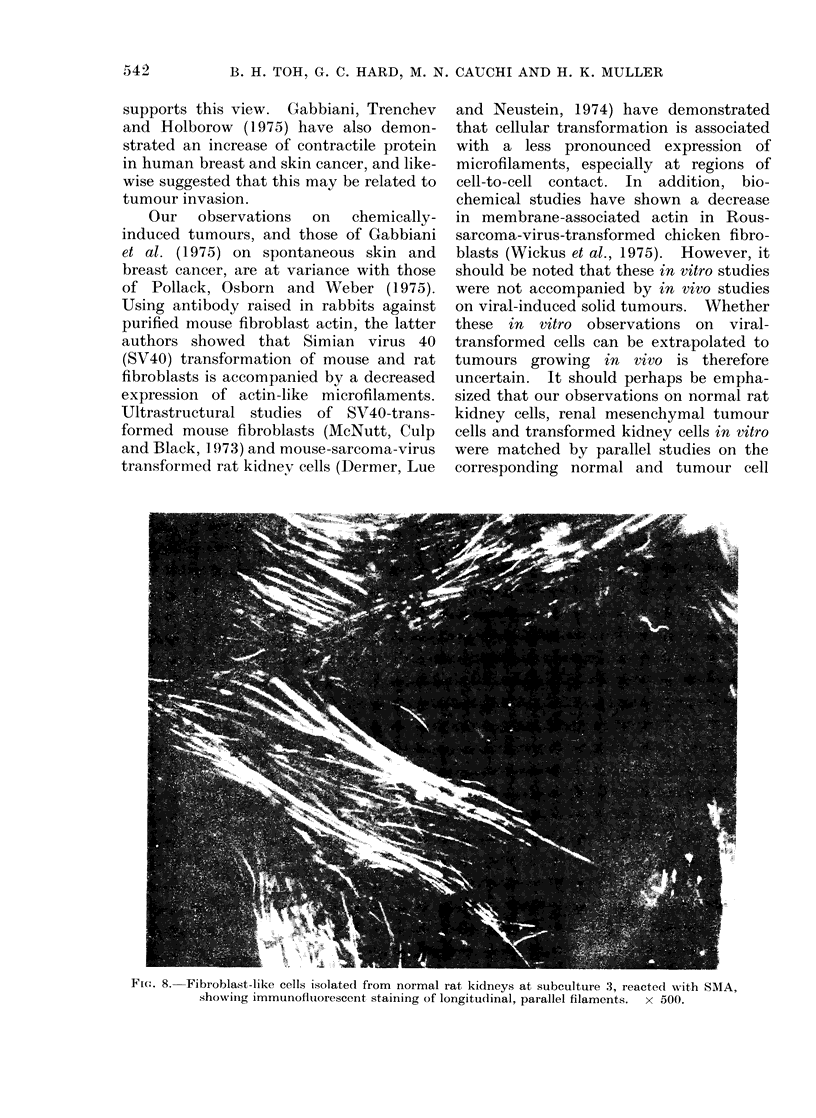
Cryostat sections and established in vitro cultures of dimethylnitrosamine(DMN)-induced renal mesenchymal tumours and monolayer cultures of transformed kidney cells derived from rats treated with a carcinogenic dose of DMN were examined by indirect immunofluorescence with human serum containing smooth muscle antibody. Eight mesenchymal tumours examined showed filamentous cytoplasmic staining of spindle cells infiltrating between renal tubules, whilst in normal kidneys interstitial cells were only weakly positive. In established in vitro cultures from 6 mesenchymal tumours, different patterns of staining were observed in morphologically different cell forms, ranging from fine filamentous staining in giant cells to diffuse cytoplasmic fluorescence in small bipolar cells, and cell outline staining in polygonal cells. In addition filamentous staining of microvillous projections and nucleolar staining were observed in some tumour cells. Monolayer cultures of transformed kidney cells showed strong staining of coarse, randomly-orientated cytoplasmic filaments, whilst fibroblasts cultured from normal rat kidney demonstrated an ordered array of fine, parallel filaments. Specificity of the immunofluorescent staining reaction was established by failure to obtain staining with normal serum, with smooth muscle antibody serum neutralized by homogenates of smooth muscle or extracts containing actin derived from smooth muscle. These results indicate that there is an apparent increase of actin-like contractile microfilaments in transformed cells and in renal mesenchymal tumours. The cytoplasmic contracile microfilaments in these cells may play a role in tumour cell mobility and invasion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanism of movement and maintenance of polarity in leucocytes. Antibiot Chemother (1971) 1974;19:191–217. doi: 10.1159/000395432. [DOI] [PubMed] [Google Scholar]
- Borland R., Hard G. C. Early appearance of "transformed" cells from the kidneys of rats treated with a "single" carcinogenic dose of dimethylnitrosamine (DMN) detected by culture in vitro. Eur J Cancer. 1974 Mar;10(3):177–184. doi: 10.1016/0014-2964(74)90151-0. [DOI] [PubMed] [Google Scholar]
- Dermer G. B., Lue J., Neustein H. B. Comparison of surface material, cytoplasmic filaments, and intercellular junctions from untransformed and two mouse sarcoma virus-transformed cell lines. Cancer Res. 1974 Jan;34(1):31–38. [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Lamelin J. P., Vassalli P., Majno G., Bouvier C. A., Cruchaud A., Lüscher E. F. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to "nonmuscular" cells. Am J Pathol. 1973 Sep;72(3):473–488. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Trenchev P., Holborow E. J. Increase of contractile proteins in human cancer cells. Lancet. 1975 Oct 25;2(7939):796–797. doi: 10.1016/s0140-6736(75)80008-0. [DOI] [PubMed] [Google Scholar]
- Hard G. C. Autoradiographic analysis of proliferative activity in rat kidney epithelial and mesenchymal cell subpopulations following a carcinogenic dose of dimethylnitrosamine. Cancer Res. 1975 Dec;35(12):3762–3773. [PubMed] [Google Scholar]
- Hard G. C., Borland R., Butler W. H. Altered morphology and behaviour of kidney fibroblasts in vitro, following in vivo treatment of rats with a carcinogenic dose of dimethylnitrosamine. Experientia. 1971 Oct 15;27(10):1208–1209. doi: 10.1007/BF02286932. [DOI] [PubMed] [Google Scholar]
- Hard G. C., Borland R. In vitro culture of cells isolate from dimethylnitrosamine-induced renal mesenchymal tumors of the rat. I. Qualitative morphology. J Natl Cancer Inst. 1975 May;54(5):1085–1095. doi: 10.1093/jnci/54.5.1085. [DOI] [PubMed] [Google Scholar]
- Hard G. C., Borland R. In vitro culture of cells isolated from dimethylnitrosamine-induced renal mesenchymal tumors of the rat. Oncology. 1974;30(6):485–492. doi: 10.1159/000224992. [DOI] [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Cellular analysis of renal neoplasia: induction of renal tumors in dietary-conditioned rats by dimethylnitrosamine, with a reappraisal of morphological characteristics. Cancer Res. 1970 Nov;30(11):2796–2805. [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Cellular analysis of renal neoplasia: light microscope study of the development of interstitial lesions induced in the rat kidney by a single carcinogenic dose of dimethylnitrosamine. Cancer Res. 1970 Nov;30(11):2806–2815. [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Ultrastructural analysis of renal mesenchymal tumor induced in the rat by dimethylnitrosamine. Cancer Res. 1971 Mar;31(3):348–365. [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Ultrastructural study of the development of interstitial lesions leading to mesenchymal neoplasia induced in the rat renal cortex by dimethylnitrosamine. Cancer Res. 1971 Mar;31(3):337–347. [PubMed] [Google Scholar]
- Heaysman J. E., Pegrum S. M. Early contacts between fibroblasts. An ultrastructural study. Exp Cell Res. 1973 Mar 30;78(1):71–78. doi: 10.1016/0014-4827(73)90039-6. [DOI] [PubMed] [Google Scholar]
- Hodson M. E., Turner-Warwick M. Autoantibodies in patients with bronchial carcinoma. Thorax. 1975 Aug;30(4):367–370. doi: 10.1136/thx.30.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Becker M., Hindennach I., Jockusch H. Slime mould actin: homology to vertebrate actin and presence in the nucleus. Exp Cell Res. 1974 Dec;89(2):241–246. doi: 10.1016/0014-4827(74)90787-3. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Holborow E. J., Glynn L. E. Antibody to smooth muscle in patients with liver disease. Lancet. 1965 Oct 30;2(7418):878–879. doi: 10.1016/s0140-6736(65)92505-5. [DOI] [PubMed] [Google Scholar]
- Lestourgeon W. M., Forer A., Yang Y. Z., Bertram J. S., Pusch H. P. Contractile proteins. Major components of nuclear and chromosome non-histone proteins. Biochim Biophys Acta. 1975 Feb 27;379(2):529–552. [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. S. Immunity to infection, allograft immunity and tumour immunity: parallels and contrasts. Transplant Rev. 1974;19(0):226–254. doi: 10.1111/j.1600-065x.1974.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precursors of ribosomal RNA. Nature. 1971 Jul 9;232(5306):86–86. doi: 10.1038/232086a0. [DOI] [PubMed] [Google Scholar]
- Rostgaard J. Electron microscopy of filaments in the basal part of rat kidney tubule cells, and their in situ interaction with heavy meromyosin. Z Zellforsch Mikrosk Anat. 1972;132(4):497–521. doi: 10.1007/BF00306638. [DOI] [PubMed] [Google Scholar]
- Rostgaard J., Thuneberg L. Electron microscopical observations on the brush border of proximal tubule cells of mammalian kidney. Z Zellforsch Mikrosk Anat. 1972;132(4):473–496. doi: 10.1007/BF00306637. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Presence of actin during chromosomal movement. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2451–2455. doi: 10.1073/pnas.72.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenberg A. E., Muller H. K., Cauchi M. N., Nairn R. C. Incidence of autoantibodies in cancer patients. Clin Exp Immunol. 1973 Oct;15(2):153–156. [PMC free article] [PubMed] [Google Scholar]
- Wasserman J., Glas U., Blomgren H. Autoantibodies in patients with carcinoma of the breast. Correlation with prognosis. Clin Exp Immunol. 1975 Mar;19(3):417–422. [PMC free article] [PubMed] [Google Scholar]
- Whitehouse J. M., Holborow E. J. Smooth muscle antibody in malignant disease. Br Med J. 1971 Nov 27;4(5786):511–513. doi: 10.1136/bmj.4.5786.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingham S., Mackay I. R., Irwin J. Autoimmune hepatitis. Immunofluorescence reactions with cytoplasm of smooth muscle and renal glomerular cells. Lancet. 1966 Jun 18;1(7451):1333–1335. doi: 10.1016/s0140-6736(66)92131-3. [DOI] [PubMed] [Google Scholar]
- Wickus G., Gruenstein E., Robbins P. W., Rich A. Decrease in membrane-associated actin of fibroblasts after transformation by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):746–749. doi: 10.1073/pnas.72.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Cyclic AMP modulates microvillus formation and agglutinability in transformed and normal mouse fibroblasts. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1263–1267. doi: 10.1073/pnas.72.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Z., Perdue J. F. Contractile proteins of cultured cells. I. The isolation and characterization of an actin-like protein from cultured chick embryo fibroblasts. J Biol Chem. 1972 Jul 25;247(14):4503–4509. [PubMed] [Google Scholar]