Abstract
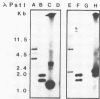
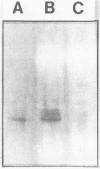
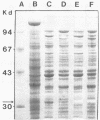
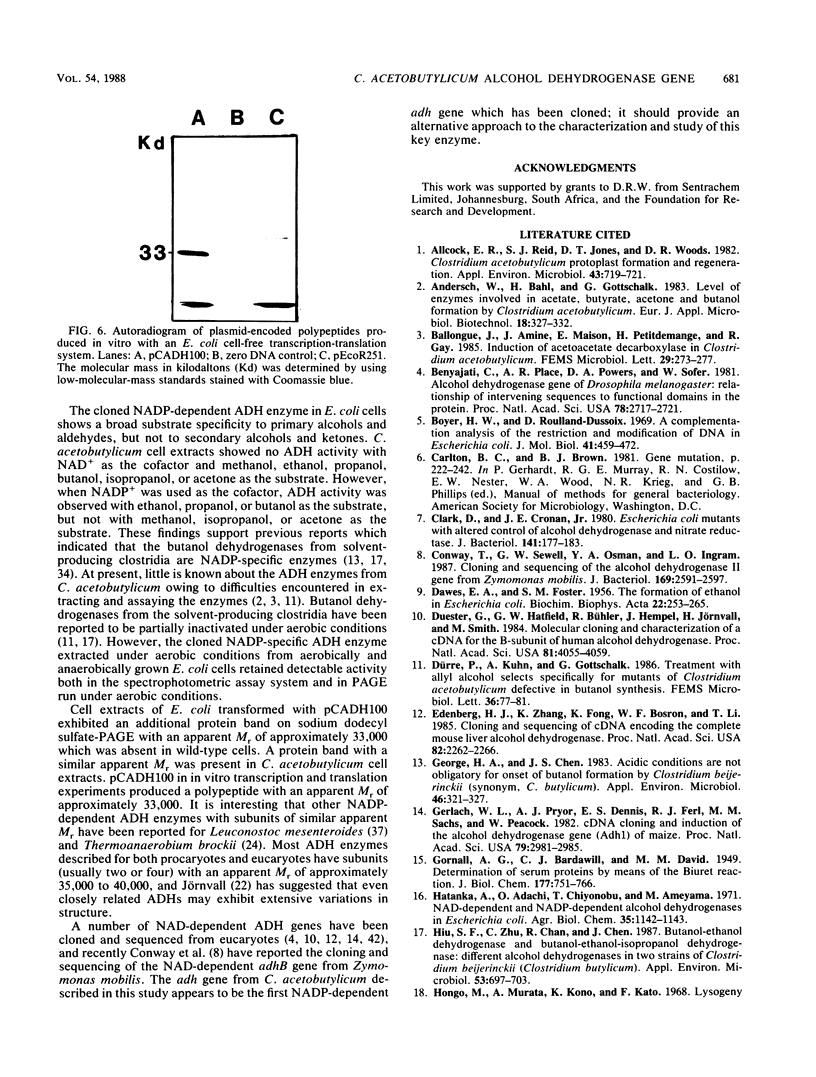
An alcohol dehydrogenase (ADH) gene from Clostridium acetobutylicum was cloned on a recombinant plasmid, pCADH100. Escherichia coli HB101, and an allyl alcohol-resistant mutant, HB101-adh1, containing this plasmid were unable to grow aerobically or anaerobically on agar media containing sublethal concentrations of allyl alcohol. E. coli HB101 and HB101-adh1 transformed with the plasmid pCADH100 produced increased levels of ethanol when grown anaerobically under alkaline conditions in the absence of nitrate. Cell extracts from aerobically and anaerobically grown E. coli HB101(pCADH100) and HB101-adhl(pCADH100) cells exhibited increased levels of NADP-dependent ADH activity with either ethanol or butanol as the substrate. The inability of E. coli HB101(pCADH100) to grow in the presence of allyl alcohol correlated with the appearance of an NADP-dependent ADH activity band on nondenaturing polyacrylamide gel electrophoresis with either ethanol or butanol as the substrate. The position of the cloned NADP-dependent ADH activity bands in E. coli HB101(pCADH100) cell extracts with either ethanol or butanol as the substrate coincided with the position of a single NADP-dependent ADH activity band in extracts of C. acetobutylicum cells. E. coli HB101(pCADH100) cell extracts prepared from both aerobically and anaerobically grown cells exhibited an additional protein band with an apparent Mr of approximately 33,000 on sodium dodecyl sulfate-polyacryl-amide gel electrophoresis which was absent in cell extracts of E. coli HB101. A protein band with a similar apparent Mr was observed in cell extracts of C. acetobutylicum, and in vitro transcription and translation experiments with pCADH100 produced a major protein product with a similar apparent Mr.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcock E. R., Reid S. J., Jones D. T., Woods D. R. Clostridium acetobutylicum Protoplast Formation and Regeneration. Appl Environ Microbiol. 1982 Mar;43(3):719–721. doi: 10.1128/aem.43.3.719-721.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Place A. R., Powers D. A., Sofer W. Alcohol dehydrogenase gene of Drosophila melanogaster: relationship of intervening sequences to functional domains in the protein. Proc Natl Acad Sci U S A. 1981 May;78(5):2717–2721. doi: 10.1073/pnas.78.5.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clark D., Cronan J. E., Jr Escherichia coli mutants with altered control of alcohol dehydrogenase and nitrate reductase. J Bacteriol. 1980 Jan;141(1):177–183. doi: 10.1128/jb.141.1.177-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Sewell G. W., Osman Y. A., Ingram L. O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987 Jun;169(6):2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., FOSTER S. M. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956 Nov;22(2):253–265. doi: 10.1016/0006-3002(56)90148-2. [DOI] [PubMed] [Google Scholar]
- Duester G., Hatfield G. W., Bühler R., Hempel J., Jörnvall H., Smith M. Molecular cloning and characterization of a cDNA for the beta subunit of human alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4055–4059. doi: 10.1073/pnas.81.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Zhang K., Fong K., Bosron W. F., Li T. K. Cloning and sequencing of cDNA encoding the complete mouse liver alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2262–2266. doi: 10.1073/pnas.82.8.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Chen J. S. Acidic Conditions Are Not Obligatory for Onset of Butanol Formation by Clostridium beijerinckii (Synonym, C. butylicum). Appl Environ Microbiol. 1983 Aug;46(2):321–327. doi: 10.1128/aem.46.2.321-327.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiu S. F., Zhu C. X., Yan R. T., Chen J. S. Butanol-Ethanol Dehydrogenase and Butanol-Ethanol-Isopropanol Dehydrogenase: Different Alcohol Dehydrogenases in Two Strains of Clostridium beijerinckii (Clostridium butylicum). Appl Environ Microbiol. 1987 Apr;53(4):697–703. doi: 10.1128/aem.53.4.697-703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., van der Westhuizen A., Long S., Allcock E. R., Reid S. J., Woods D. R. Solvent Production and Morphological Changes in Clostridium acetobutylicum. Appl Environ Microbiol. 1982 Jun;43(6):1434–1439. doi: 10.1128/aem.43.6.1434-1439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Differences between alcohol dehydrogenases. Structural properties and evolutionary aspects. Eur J Biochem. 1977 Feb;72(3):443–452. doi: 10.1111/j.1432-1033.1977.tb11268.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorowitz W., Clark D. Escherichia coli mutants with a temperature-sensitive alcohol dehydrogenase. J Bacteriol. 1982 Nov;152(2):935–938. doi: 10.1128/jb.152.2.935-938.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Allyl alcohol-induced irreversible inhibition of yeast alcohol dehydrogenase. Biochem Pharmacol. 1974 Aug 15;23(16):2328–2331. doi: 10.1016/0006-2952(74)90563-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacylamide gel electrophoresis. Anal Biochem. 1971 Mar;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- Rudolph F. B., Purich D. L., Fromm H. J. Coenzyme A-linked aldehyde dehydrogenase from Escherichia coli. I. Partial purification, properties, and kinetic studies of the enzyme. J Biol Chem. 1968 Nov 10;243(21):5539–5545. [PubMed] [Google Scholar]
- Schmitt B. Aldehyde dehydrogenase activity of a complex particle from E. coli. Biochimie. 1975;57(9):1001–1004. doi: 10.1016/s0300-9084(75)80355-5. [DOI] [PubMed] [Google Scholar]
- Schneider-Bernlöhr H., Fiedler H., Gerber M., Weber C., Zeppezauer M. Alcohol dehydrogenase from Leuconostoc mesenteroides: molecular properties in comparison with the yeast and horse liver enzyme. Int J Biochem. 1981;13(12):1215–1224. doi: 10.1016/0020-711x(81)90067-7. [DOI] [PubMed] [Google Scholar]
- Singer M. E., Finnerty W. R. Alcohol dehydrogenases in Acinetobacter sp. strain HO1-N: role in hexadecane and hexadecanol metabolism. J Bacteriol. 1985 Dec;164(3):1017–1024. doi: 10.1128/jb.164.3.1017-1024.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Williamson V. M., Bennetzen J., Young E. T., Nasmyth K., Hall B. D. Isolation of the structural gene for alcohol dehydrogenase by genetic complementation in yeast. Nature. 1980 Jan 10;283(5743):214–216. doi: 10.1038/283214a0. [DOI] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe H., Jones D. T., Woods D. R. Cloning and expression of Clostridium acetobutylicum endoglucanase, cellobiase and amino acid biosynthesis genes in Escherichia coli. J Gen Microbiol. 1986 May;132(5):1367–1372. doi: 10.1099/00221287-132-5-1367. [DOI] [PubMed] [Google Scholar]