Abstract
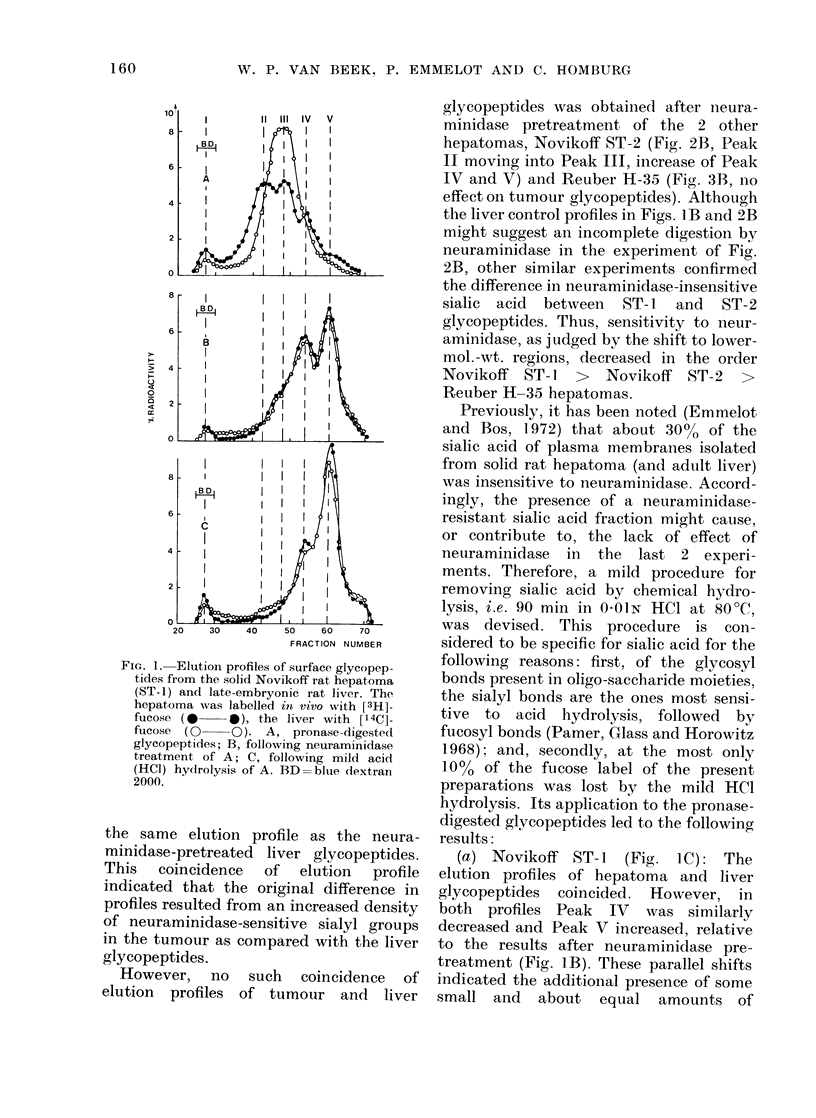
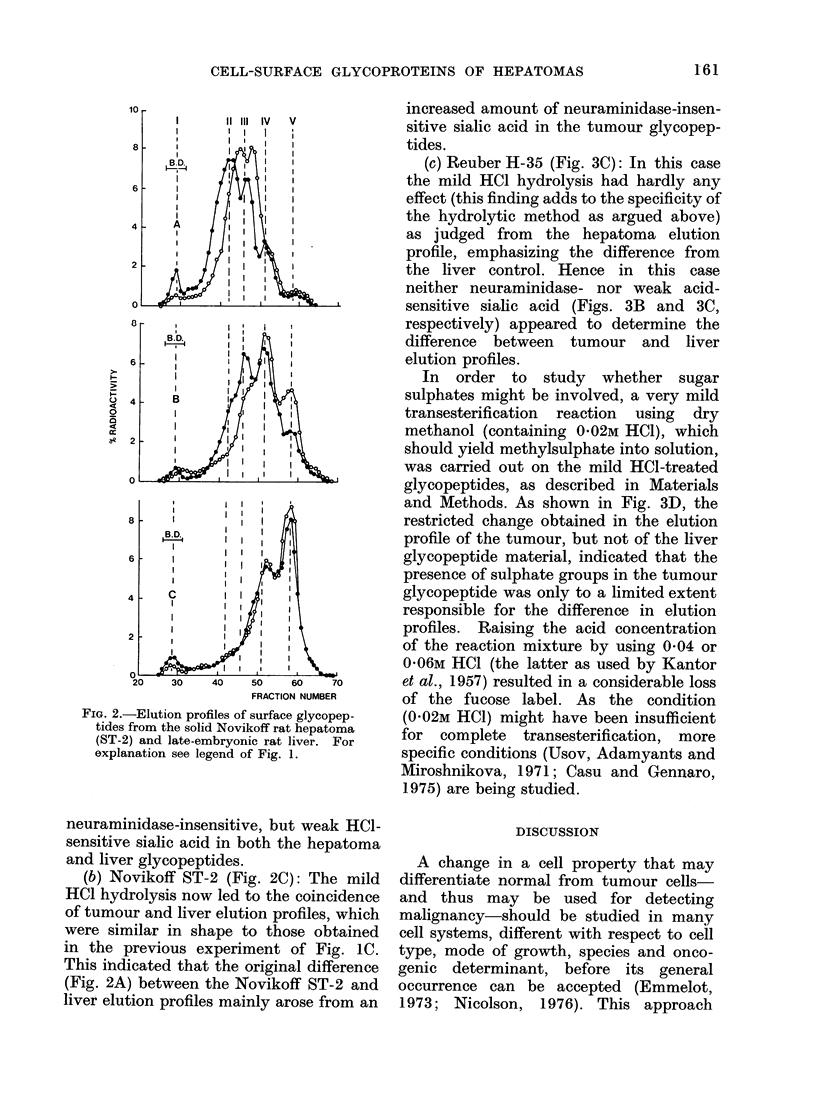
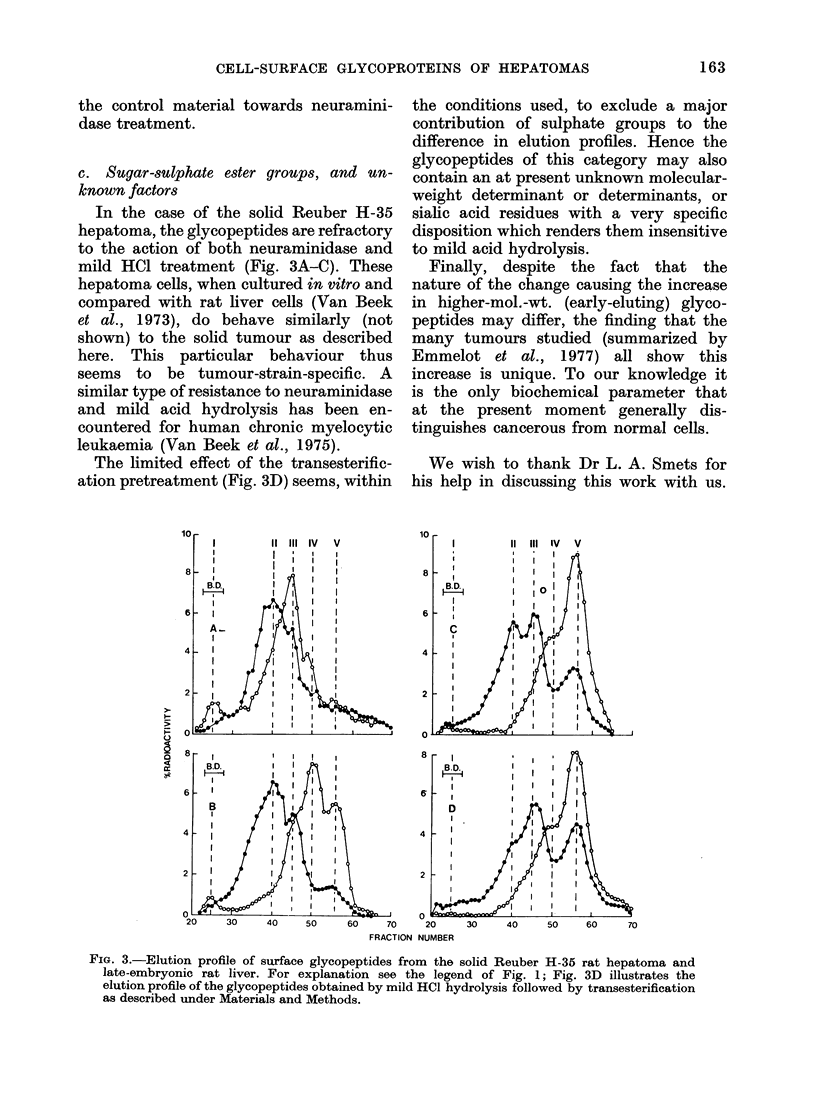
Cell-surface glycoprotein of 3 rat hepatoma strains and late-embryonic liver was metabolically labelled in vivo with [3H]- or [14C]-fucose. Trypsinization of the cells and exhaustive pronase digestion of combined hepatoma-liver trypsinates followed by gel filtration over Sephadex-Biogel mixtures, yielded elution profiles that contained more early-eluting (high-mol.-wt.) glycopeptides for hepatomas than for liver. At least 3 factors were identified which acted to augment the fraction of early-eluting tumour glycopeptides: (a) increase of neuraminidase-sensitive sialic acid, (b) increase of neuraminidase-insensitive sialic acid that was sensitive to mild HCl hydrolysis, and (c) presence of sugar sulphate groups contributing to a restricted extent, relative to possible unknown factor(s). Whether (a), (b) or (c) operated depended on the hepatoma strain or its mode of growth. Notwithstanding these differences in the nature of the increase in early-eluting glycopeptides, the increase itself appears not to be due to growth per se, nor to an embryonic expression, but rather may serve as a marker of tumourigenicity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasaki M., Kawasaki T., Yamashina I. The isolation and characterization of glycopeptides and mucopolysaccharides from plasma membranes of normal and regenerating livers of rats. FEBS Lett. 1975 Nov 1;59(1):100–104. doi: 10.1016/0014-5793(75)80350-4. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H., Summers D. F. Purification and properties of HeLa cell plasma membranes. J Biol Chem. 1971 Aug 25;246(16):5162–5175. [PubMed] [Google Scholar]
- Baldwin R. W. Immunological aspects of chemical carcinogenesis. Adv Cancer Res. 1973;18:1–75. doi: 10.1016/s0065-230x(08)60750-2. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Glycopeptides from the surface of control and virus-transformed cells. Science. 1971 Apr 9;172(3979):169–171. doi: 10.1126/science.172.3979.169. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK G. M., SEAMAN G. V., WEISS L. PHYSICOCHEMICAL DIFFERENCES BETWEEN ASCITIC AND SOLID FORMS OF SARCOMA 37 CELLS. Cancer Res. 1963 Dec;23:1813–1818. [PubMed] [Google Scholar]
- Carney P. G., Malmgren R. A. Comparison of techniques for obtaining single cell suspensions from tumors. Transplantation. 1967 May;5(3):455–458. doi: 10.1097/00007890-196705000-00007. [DOI] [PubMed] [Google Scholar]
- Casu B., Gennaro U. A conductimetric method for the determination of sulphate and carboxyl groups in heparin and other mucopolysaccharides. Carbohydr Res. 1975 Jan;39(1):168–176. doi: 10.1016/s0008-6215(00)82654-3. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Emmelot P. Biochemical properties of normal and neoplastic cell surfaces; a review. Eur J Cancer. 1973 May;9(5):319–333. doi: 10.1016/0014-2964(73)90047-9. [DOI] [PubMed] [Google Scholar]
- Glick M. C., Rabinowitz Z., Sachs L. Surface membrane glycopeptides correlated with tumorigenesis. Biochemistry. 1973 Nov 20;12(24):4864–4869. doi: 10.1021/bi00748a009. [DOI] [PubMed] [Google Scholar]
- Glick M. C., Rabinowitz Z., Sachs L. Surface membrane glycopeptides which coincide with virus transformation and tumorigenesis. J Virol. 1974 May;13(5):967–974. doi: 10.1128/jvi.13.5.967-974.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Kojima K., Maekawa A. A difference in the architecture of surface membrane between two cell types of rat ascites hepatomas. Cancer Res. 1972 Apr;32(4):847–852. [PubMed] [Google Scholar]
- Kojima K., Maekawa A. Difference in electrokinetic charge of cells between two cell types of ascites hepatoma after removal of sialic acid. Cancer Res. 1970 Dec;30(12):2858–2862. [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Giovanella B. C., Stehlin J. S., Klein G. Tumorigenicity of human hematopoietic cell lines in athymic nude mice. Int J Cancer. 1977 Mar 15;19(3):337–344. doi: 10.1002/ijc.2910190309. [DOI] [PubMed] [Google Scholar]
- Ogata S. I., Muramatsu T., Kobata A. New structural characteristic of the large glycopeptides from transformed cells. Nature. 1976 Feb 19;259(5544):580–582. doi: 10.1038/259580a0. [DOI] [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C., MORSE P. A., Jr, POTTER V. R. HEPATOMAS IN TISSUE CULTURE COMPARED WITH ADAPTING LIVER IN VIVO. Natl Cancer Inst Monogr. 1964 Apr;13:229–245. [PubMed] [Google Scholar]
- Pamer T., Glass G. B., Horowitz M. I. Purification and characterization of sulfated glycoproteins and hyaluronidase-resistant mucopolysaccharides from dog gastric mucosa. Biochemistry. 1968 Nov;7(11):3821–3829. doi: 10.1021/bi00851a006. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets L. A., van Beek W. P., van Rooij H. Surface glycoproteins and concanavalin-A-mediated agglutinability of clonal variants and tumour cells derived from SV40-virus-transformed mouse 3T3 cells. Int J Cancer. 1976 Oct 15;18(4):462–468. doi: 10.1002/ijc.2910180411. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Van Beek W. P., Smets L. A., Emmelot P. Changed surface glycoprotein as a marker of malignancy in human leukaemic cells. Nature. 1975 Feb 6;253(5491):457–460. doi: 10.1038/253457a0. [DOI] [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of normal and transformed cells: a difference determined by sialic acid and a growth-dependent sialyl transferase. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1838–1842. doi: 10.1073/pnas.69.7.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L., Zeidman I., Buck C. A. The surface glycoproteins of a mouse melanoma growing in culture and as a solid tumor in vivo. Cancer Res. 1975 Aug;35(8):2186–2190. [PubMed] [Google Scholar]
- van Beek W. P., Smets L. A., Emmelot P. Increased sialic acid density in surface glycoprotein of transformed and malignant cells--a general phenomenon? Cancer Res. 1973 Nov;33(11):2913–2922. [PubMed] [Google Scholar]


