Abstract
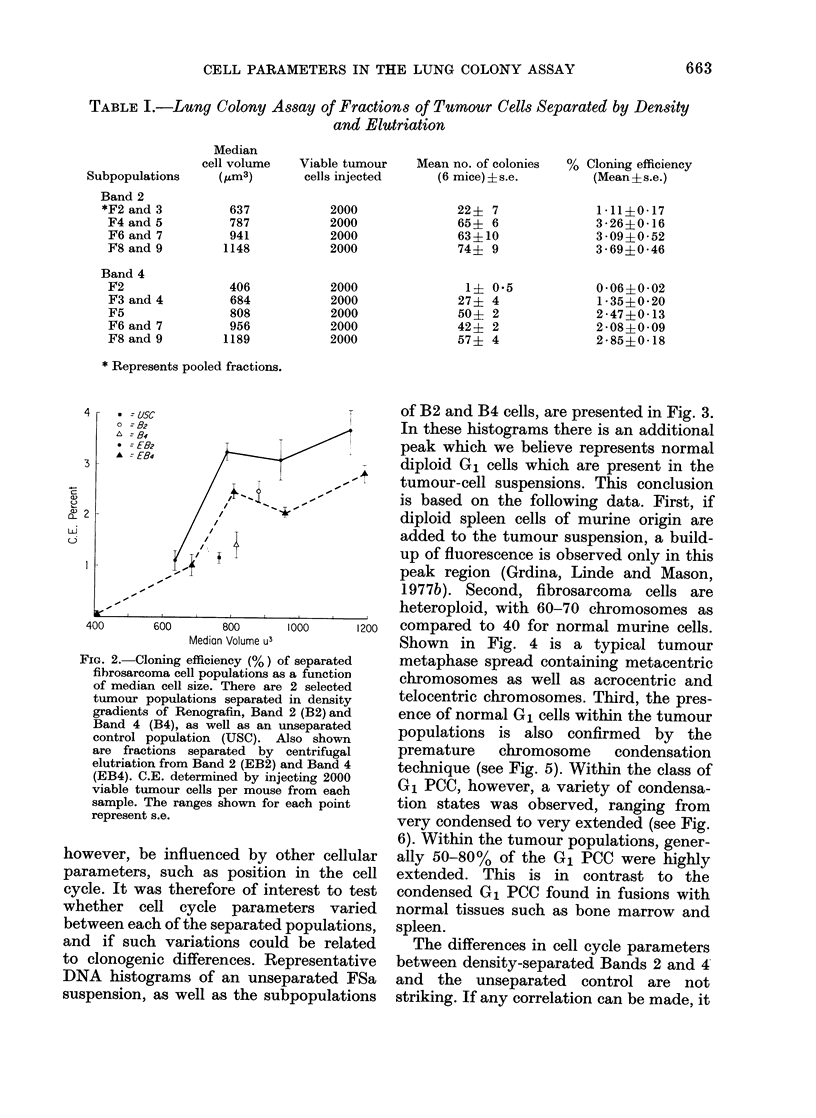
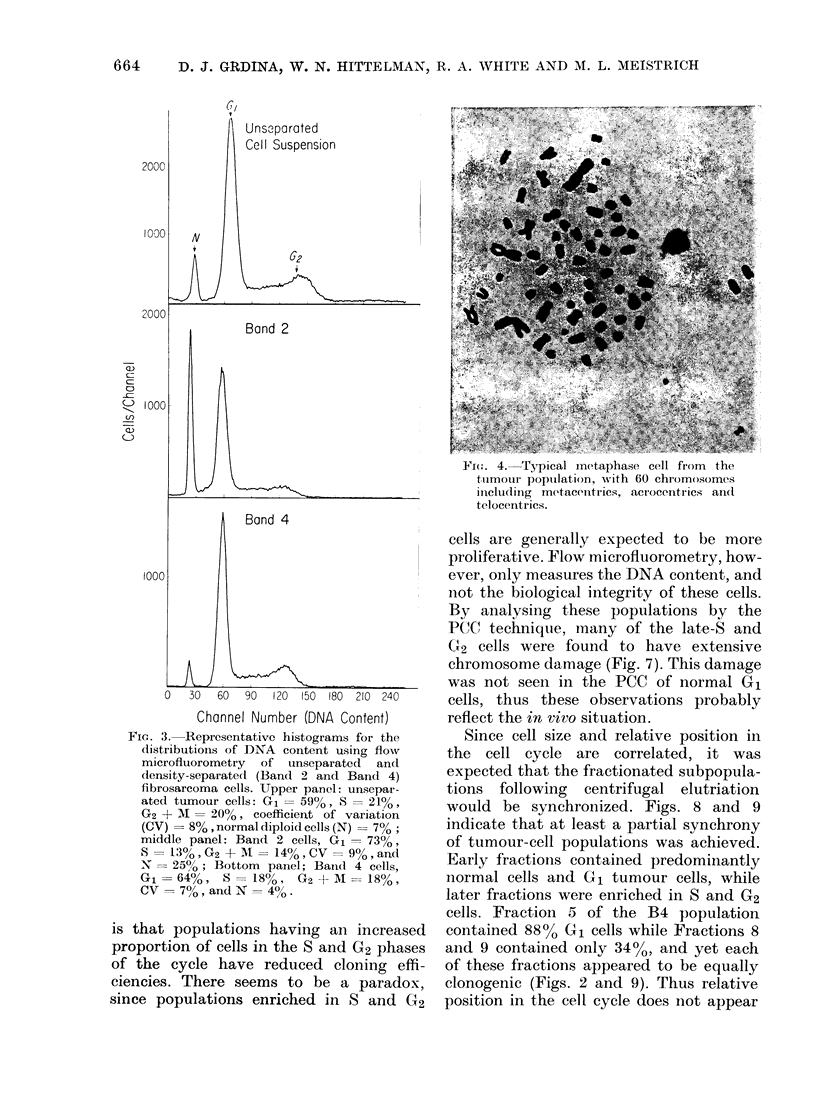
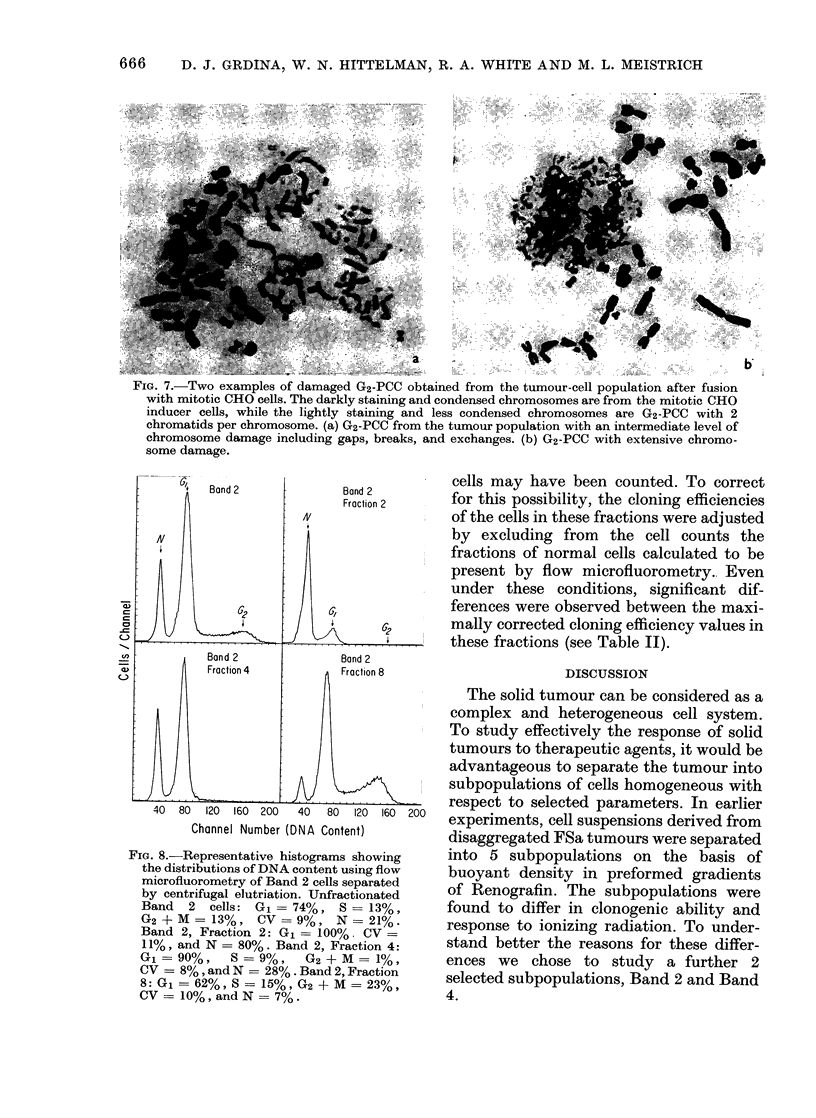
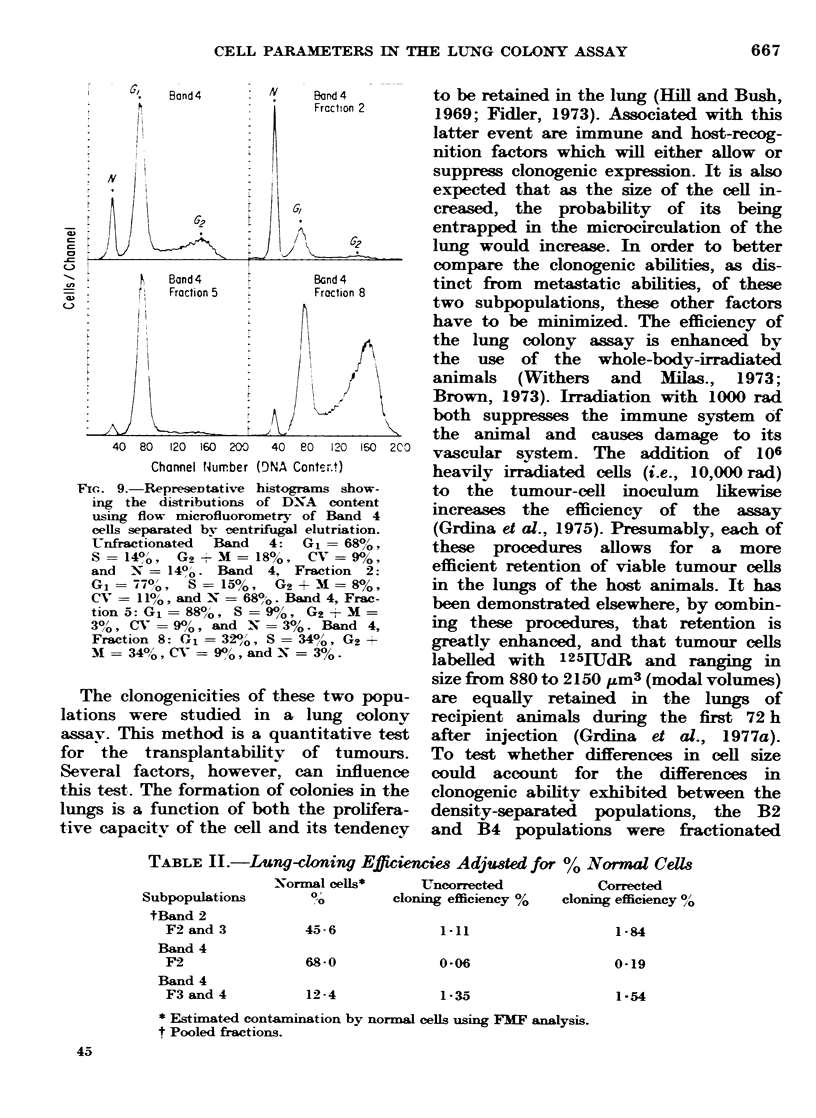
Mouse fibrosarcoma tumours were dissociated and divided into subpopulations of viable cells by centrifugation in linear density gradients of Renografin. Two of these subpopulations, designated Band 2 and Band 4, differed in their clonogenic ability in lung colony assay. The less dense Band 2 cells were significantly more clonogenic than the Band 4 cells (2.9 percent vs 1.4 percent respectively). Each band was further separated on the basis of cell size by centrifugal elutriation. Each size class of cells comprising Band 2 showed higher clonogenic ability than the corresponding size class in Band 4. Thus cell size differences were not responsible for the clonogenic differences between these bands. To determine whether cell-cycle distribution of the tumour cells was responsible for differences in cloning efficiency, flow microfluorometric and premature chromosome condensation methods were utilized. The unseparated and Band 4 populations showed a higher percentage of cells in S and G2 than did the Band 2 populations, but many of the S and G2 tumour cells showed extensive chromosome damage. From this study we conclude that the increased clonogenic ability of the lighter tumour cells is not due to differences in cell size or cell-cycle parameters.
Full text
PDF


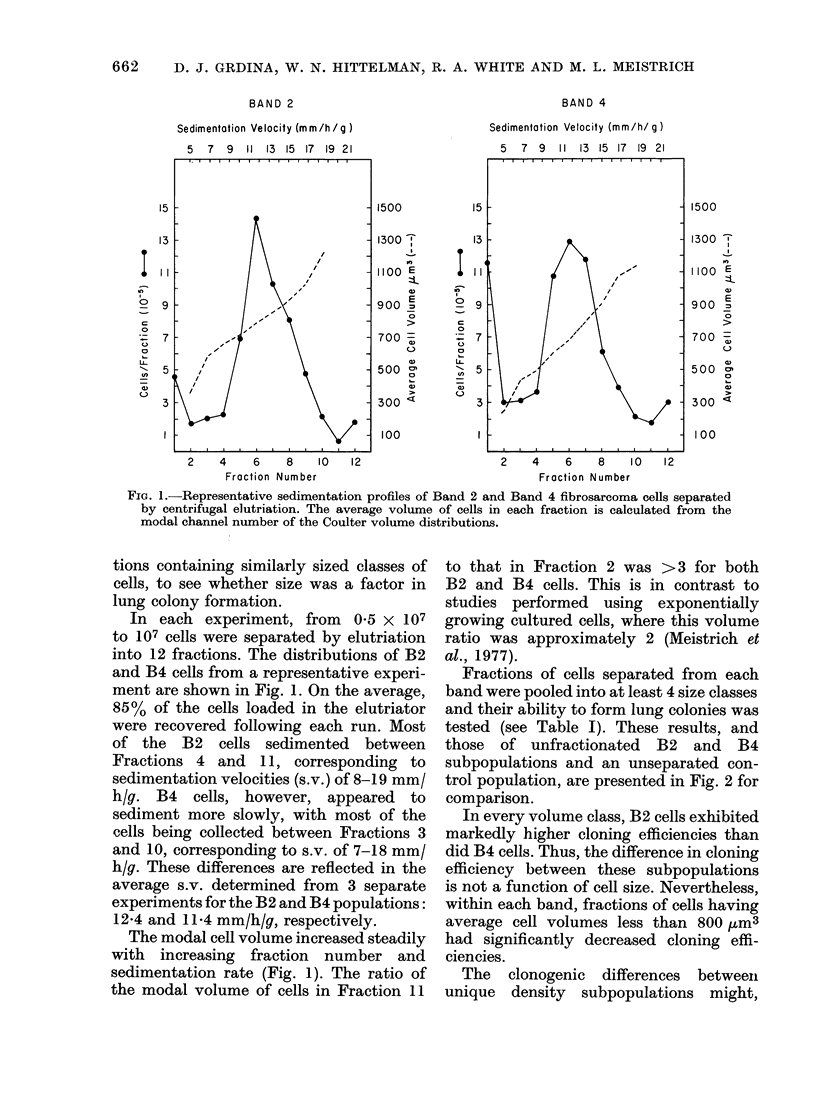



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlogie B., Drewinko B., Johnston D. A., Büchner T., Hauss W. H., Freireich E. J. Pulse cytophotometric analysis of synchronized cells in vitro. Cancer Res. 1976 Mar;36(3):1176–1181. [PubMed] [Google Scholar]
- Bhambhani R., Kuspira J., Giblak R. E. A comparison of cell survival and chromosomal damage using CHO cells synchronized with and without colcemid. Can J Genet Cytol. 1973 Sep;15(3):605–618. doi: 10.1139/g73-072. [DOI] [PubMed] [Google Scholar]
- Brown J. M. The effect of lung irradiation on the incidence of pulmonary metastases in mice. Br J Radiol. 1973 Aug;46(548):613–618. doi: 10.1259/0007-1285-46-548-613. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973 Mar;9(3):223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- Glick D., Von Redlich D., Juhos E. T., McEwen C. R. Separation of mast cells by centrifugal elutriation. Exp Cell Res. 1971 Mar;65(1):23–26. doi: 10.1016/s0014-4827(71)80045-9. [DOI] [PubMed] [Google Scholar]
- Grdina D. J., Basic I., Guzzino S., Mason K. A. Radiation response of cell populations irradiated in situ and separated from a fibrosarcoma. Radiat Res. 1976 Jun;66(3):634–643. [PubMed] [Google Scholar]
- Grdina D. J., Basic I., Mason K. A., Withers H. R. Radiation response of clonogenic cell populations separated from fibrosarcoma. Radiat Res. 1975 Sep;63(3):483–493. [PubMed] [Google Scholar]
- Grdina D. J., Meistrich M. L., Withers H. R. Separation of clonogenic cells from stationary phase cultures by density gradient centrifugation. Exp Cell Res. 1974 Mar 30;85(1):15–22. doi: 10.1016/0014-4827(74)90207-9. [DOI] [PubMed] [Google Scholar]
- Grdina D. J., Milas L., Hewitt R. R., Withers H. R. Buoyant density separation of human blood cells in renografin gradients. Exp Cell Res. 1973 Sep;81(1):250–254. doi: 10.1016/0014-4827(73)90131-6. [DOI] [PubMed] [Google Scholar]
- Hill R. P., Bush R. S. A lung-colony assay to determine the radiosensitivity of cells of a solid tumour. Int J Radiat Biol Relat Stud Phys Chem Med. 1969 Jul;15(5):435–444. doi: 10.1080/09553006914550721. [DOI] [PubMed] [Google Scholar]
- Hittelman W. N., Rao P. N. Premature chromosome condensation. Conformational changes of chromatin associated with phytohemagglutinin stimulation of peripheral lymphocytes. Exp Cell Res. 1976 Jul;100(2):219–222. doi: 10.1016/0014-4827(76)90140-3. [DOI] [PubMed] [Google Scholar]
- Hittelman W. N., Rao P. N. Premature chromosome condensation. I. Visualization of x-ray-induced chromosome damage in interphase cells. Mutat Res. 1974 May;23(2):251–258. doi: 10.1016/0027-5107(74)90145-6. [DOI] [PubMed] [Google Scholar]
- Milas L., Tomljanovic M. Spleen colony-forming capacity of bone marrow from mice bearing fibrosarcoma. Rev Eur Etud Clin Biol. 1971 May;16(5):462–465. [PubMed] [Google Scholar]
- Rao A. P., Rao P. N. The cause of G2-arrest in Chinese hamster ovary cells treated with anticancer drugs. J Natl Cancer Inst. 1976 Nov;57(5):1139–1143. doi: 10.1093/jnci/57.5.1139. [DOI] [PubMed] [Google Scholar]
- Steinkamp J. A., Fulwyler M. J., Coulter J. R., Hiebert R. D., Horney J. L., Mullancy P. F. A new multiparameter separator for microscopic particles and biological cells. Rev Sci Instrum. 1973 Sep;44(9):1301–1310. doi: 10.1063/1.1686375. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature. 1975 Mar 20;254(5497):245–247. doi: 10.1038/254245a0. [DOI] [PubMed] [Google Scholar]
- Withers H. R., Milas L. Influence of preirradiation of lung on development of artificial pulmonary metastases of fibrosarcoma in mice. Cancer Res. 1973 Aug;33(8):1931–1936. [PubMed] [Google Scholar]