Abstract
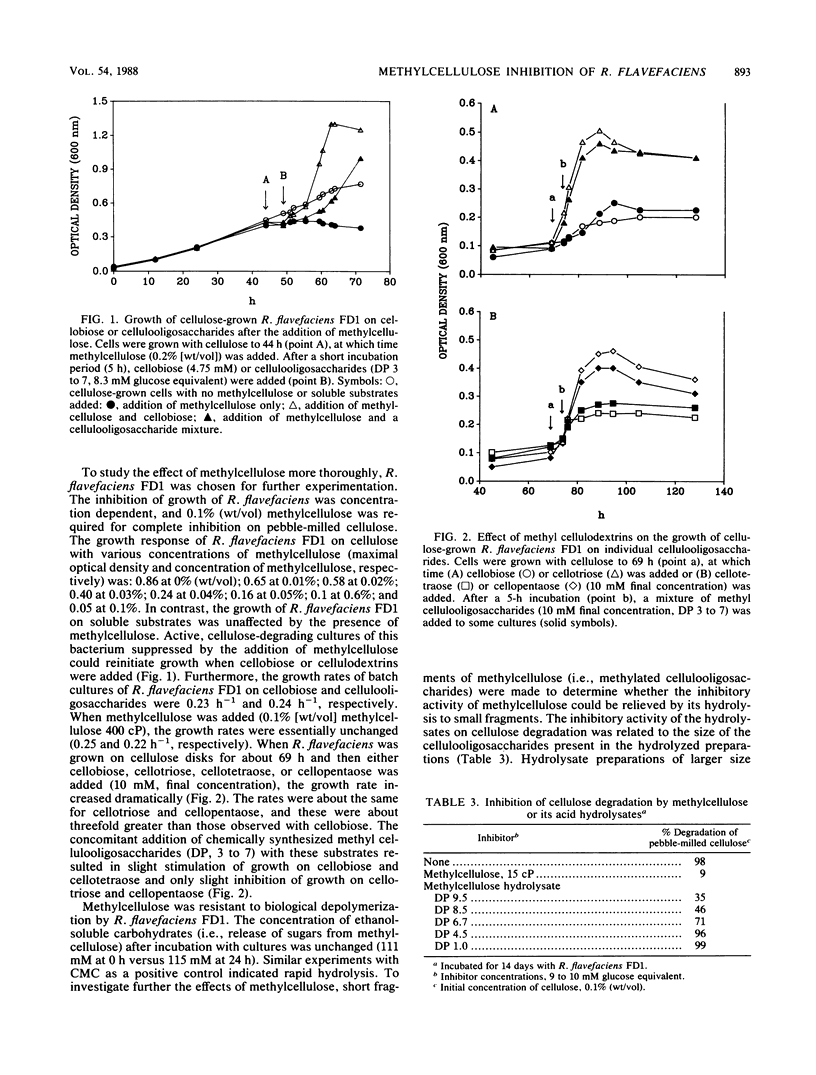
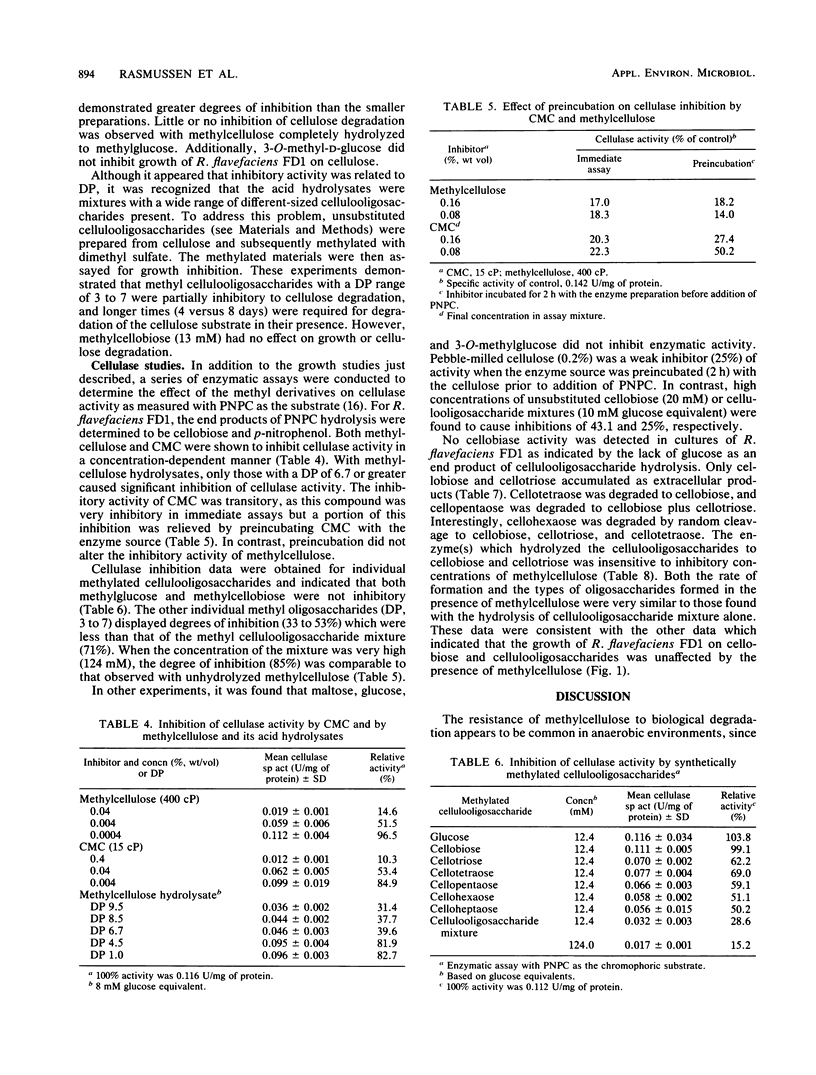
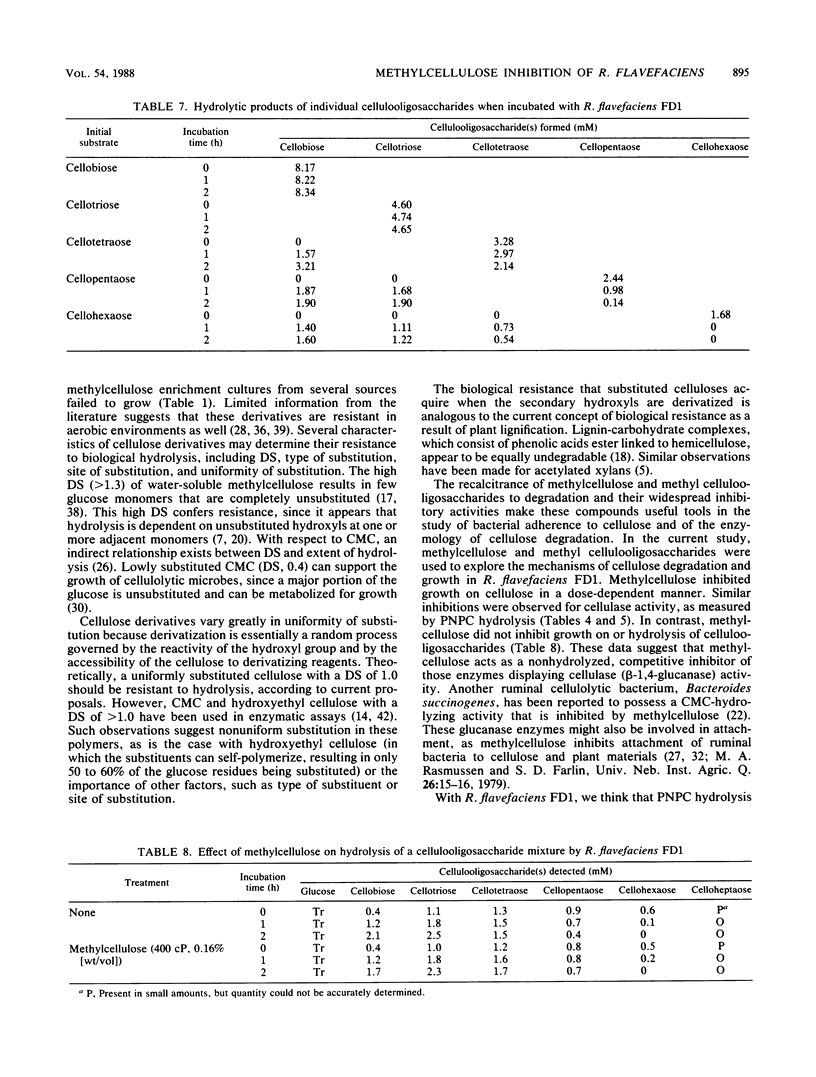
Highly methylated, long-chain celluloses strongly inhibited cellulose degradation by several species of cellulolytic bacteria of ruminal origin. Specifically, the inhibitory effects of methylcellulose on the growth of Ruminococcus flavefaciens FD1 were concentration dependent, with complete inhibition at 0.1% (wt/vol). However, methylcellulose did not inhibit growth on cellobiose or cellulooligosaccharides. Mixtures of methylated cellulooligosaccharides having an average degree of polymerization of 6.7 to 9.5 inhibited cellulose degradation, but those with an average degree of polymerization of 1.0 to 4.5 did not. Similar inhibitory effects by methylcellulose and, to a lesser extent, by methyl cellulooligosaccharides were observed on cellulase activity, as measured by hydrolysis of p-nitrophenyl-β-d-cellobioside. R. flavefaciens cultures hydrolyzed cellulooligosaccharides to cellobiose and cellotriose as final end products. Cellopentaose and cellohexaose were cleaved to these end products, but cellotetraose was also formed from cellohexaose. Methylcellulose did not inhibit hydrolysis of cellulooligosaccharides. These data are consistent with the presence of separate cellulase (β-1,4-glucanase) and cellulodextrinase activities in R. flavefaciens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYERS W. A. Phosphorolysis and synthesis of cellobiose by cell extracts from Ruminococcus flavefaciens. J Biol Chem. 1959 Nov;234:2819–2822. [PubMed] [Google Scholar]
- AYERS W. A. Phosphorylation of cellobiose and glucose by Ruminococcus flavefaciens. J Bacteriol. 1958 Nov;76(5):515–517. doi: 10.1128/jb.76.5.515-517.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., ROBINSON I. M. Characteristics of ruminal anaerobic celluloytic cocci and Cillobacterium cellulosolvens n. sp. J Bacteriol. 1958 Nov;76(5):529–537. doi: 10.1128/jb.76.5.529-537.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros M. E., Thomson J. A. Cloning and expression in Escherichia coli of a cellulase gene from Ruminococcus flavefaciens. J Bacteriol. 1987 Apr;169(4):1760–1762. doi: 10.1128/jb.169.4.1760-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Child J. J., Eveleigh D. E., Sieben A. S. Determination of cellulase activity using hydroxyethylcellulose as substrate. Can J Biochem. 1973 Jan;51(1):39–43. doi: 10.1139/o73-006. [DOI] [PubMed] [Google Scholar]
- Deshpande M. V., Eriksson K. E., Pettersson L. G. An assay for selective determination of exo-1,4,-beta-glucanases in a mixture of cellulolytic enzymes. Anal Biochem. 1984 May 1;138(2):481–487. doi: 10.1016/0003-2697(84)90843-1. [DOI] [PubMed] [Google Scholar]
- Gaillard B. D., Richards G. N. Presence of soluble lignin-carbohydrate complexes in the bovine rumen. Carbohydr Res. 1975 Jun;42(1):135–145. doi: 10.1016/s0008-6215(00)84106-3. [DOI] [PubMed] [Google Scholar]
- Gardner R. M., Doerner K. C., White B. A. Purification and characterization of an exo-beta-1,4-glucanase from Ruminococcus flavefaciens FD-1. J Bacteriol. 1987 Oct;169(10):4581–4588. doi: 10.1128/jb.169.10.4581-4588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Partial characterization of the extracellular carboxymethylcellulase activity produced by the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1983 May;29(5):504–517. doi: 10.1139/m83-080. [DOI] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. doi: 10.1128/aem.53.5.1034-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOP W., KOOIMAN P. THE ACTION OF CELLULOLYTIC ENZYMES ON SUBSTITUTED CELLULOSES. Biochim Biophys Acta. 1965 Apr 26;99:102–120. doi: 10.1016/s0926-6593(65)80011-x. [DOI] [PubMed] [Google Scholar]
- Kudo H., Cheng K. J., Costerton J. W. Electron microscopic study of the methylcellulose-mediated detachment of cellulolytic rumen bacteria from cellulose fibers. Can J Microbiol. 1987 Mar;33(3):267–272. doi: 10.1139/m87-045. [DOI] [PubMed] [Google Scholar]
- LEVINSON H. S., REESE E. T. Enzymatic hydrolysis of soluble cellulose derivatives as measured by changes in viscosity. J Gen Physiol. 1950 May 20;33(5):601–628. doi: 10.1085/jgp.33.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya K., Shimizu M., Taya M., Shimizu S. Purification and properties of cellobiosidase from Ruminococcus albus. J Bacteriol. 1982 Apr;150(1):407–409. doi: 10.1128/jb.150.1.407-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REESE E. T., SIU R. G. H., LEVINSON H. S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol. 1950 Apr;59(4):485–497. doi: 10.1128/jb.59.4.485-497.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. Fermentation of cellodextrins by cellulolytic and noncellulolytic rumen bacteria. Appl Environ Microbiol. 1985 Mar;49(3):572–576. doi: 10.1128/aem.49.3.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M. Maintenance of Laboratory strains of obligately anaerobic rumen bacteria. Appl Environ Microbiol. 1982 Aug;44(2):499–501. doi: 10.1128/aem.44.2.499-501.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


