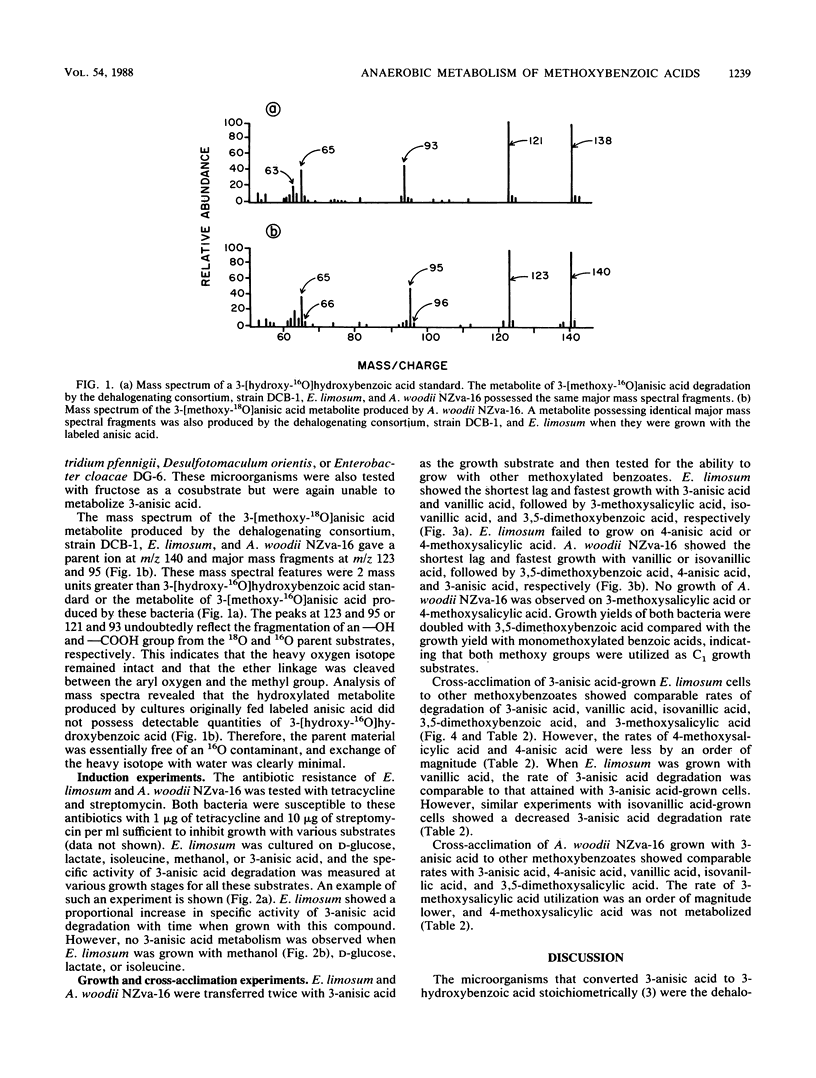
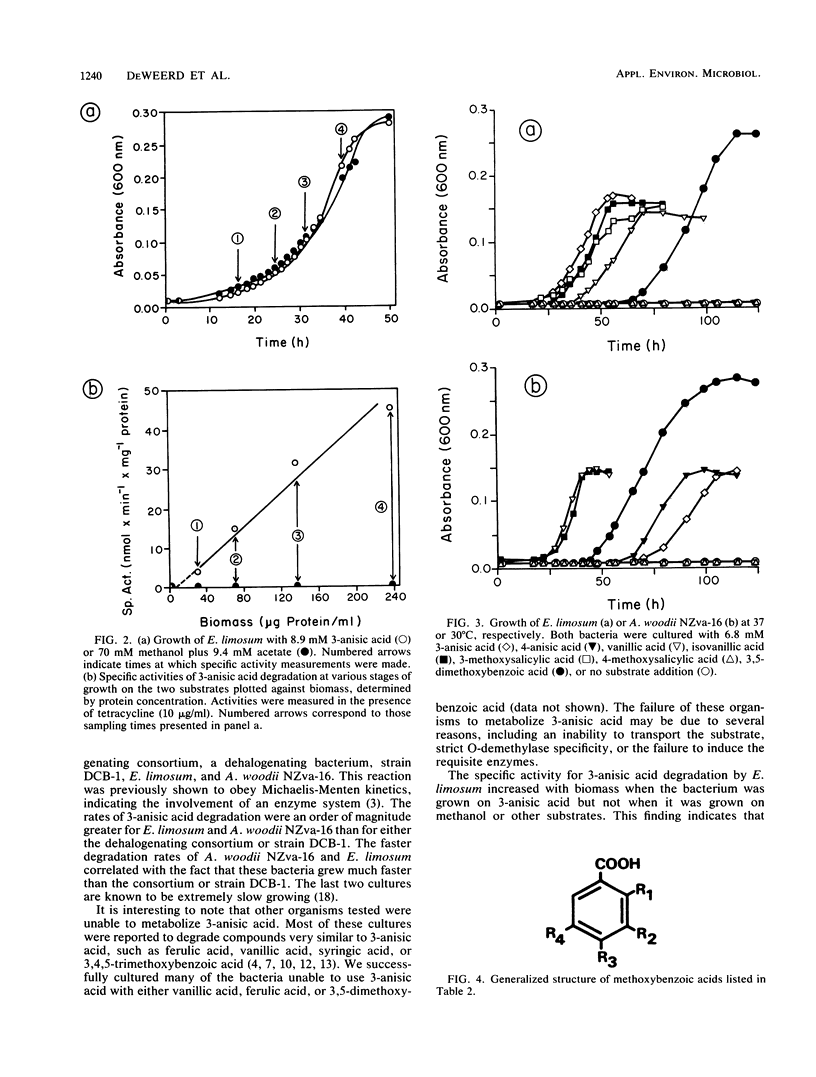
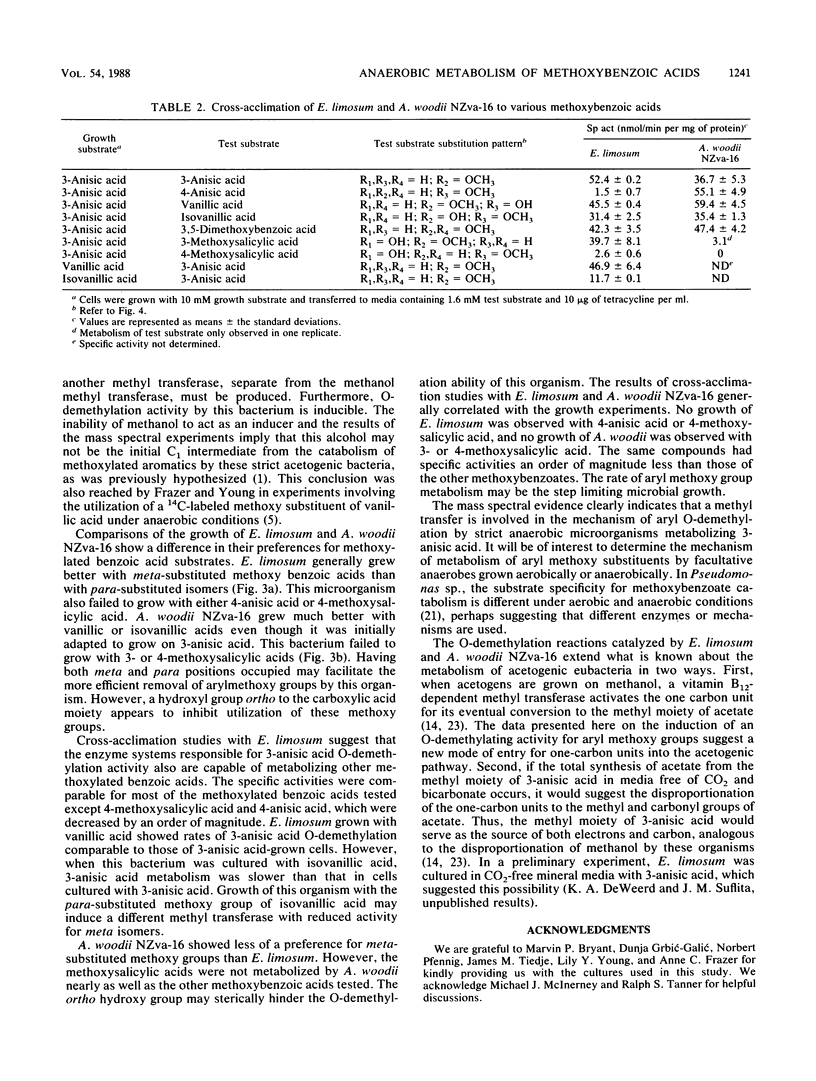
Abstract
O-methyl substituents of aromatic compounds can provide C1 growth substrates for facultative and strict anaerobic bacteria isolated from diverse environments. The mechanism of the bioconversion of methoxylated benzoic acids to the hydroxylated derivatives was investigated with a model substrate and cultures of one anaerobic consortium, eight strict anaerobic bacteria, and one facultative anaerobic microorganism. Using high-pressure liquid chromatography and gas chromatography-mass spectral analysis, we found that a haloaromatic dehalogenating consortium, a dehalogenating isolate from that consortium, Eubacterium limosum, and a strain of Acetobacterium woodii metabolized 3-[methoxy-18O]methoxybenzoic acid (3-anisic acid) to 3-[hydroxy-18O]hydroxybenzoic acid stoichiometrically at rates of 1.5, 3.2, 52.4, and 36.7 nmol/min per mg of protein, respectively. A different strain of Acetobacterium and strains of Syntrophococcus, Clostridium, Desulfotomaculum, Enterobacter, and an anaerobic bacterium, strain TH-001, were unable to transform this compound. The O-demethylating ability of E. limosum was induced only with appropriate methoxylated benzoates but not with D-glucose, lactate, isoleucine, or methanol. Cross-acclimation and growth experiments with E. limosum showed a rate of metabolism that was an order of magnitude slower and showed no growth with either 4-methoxysalicylic acid (2-hydroxy-4-methoxybenzoic acid) or 4-anisic acid (4-methoxybenzoic acid) when adapted to 3-anisic acid. However, A. woodii NZva-16 showed slower rates and no growth with 3- or 4-methoxysalicylic acid when adapted to 3-anisic acid in similar experiments. The results clearly indicate a methyl rather than methoxy group removal mechanism for such reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frazer A. C., Young L. Y. A gram-negative anaerobic bacterium that utilizes o-methyl substituents of aromatic acids. Appl Environ Microbiol. 1985 May;49(5):1345–1347. doi: 10.1128/aem.49.5.1345-1347.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Young L. Y. Anaerobic c(1) metabolism of the o-methyl-C-labeled substituent of vanillate. Appl Environ Microbiol. 1986 Jan;51(1):84–87. doi: 10.1128/aem.51.1.84-87.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbić-Galić D. O-demethylation, dehydroxylation, ring-reduction and cleavage of aromatic substrates by Enterobacteriaceae under anaerobic conditions. J Appl Bacteriol. 1986 Dec;61(6):491–497. doi: 10.1111/j.1365-2672.1986.tb01721.x. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Sharak Genthner B. R., Bryant M. P. Additional characteristics of one-carbon-compound utilization by Eubacterium limosum and Acetobacterium woodii. Appl Environ Microbiol. 1987 Mar;53(3):471–476. doi: 10.1128/aem.53.3.471-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Taylor B. F. Aerobic and Anaerobic Catabolism of Vanillic Acid and Some Other Methoxy-Aromatic Compounds by Pseudomonas sp. Strain PN-1. Appl Environ Microbiol. 1983 Dec;46(6):1286–1292. doi: 10.1128/aem.46.6.1286-1292.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


